A dentate gyrus-CA3 inhibitory circuit promotes evolution of hippocampal-cortical ensembles during memory consolidation
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This paper will be of interest to scientists across systems neuroscience or to those interested in how one component of a neural circuit contributes to downstream functions longitudinally. This study investigates how increasing feed forward inhibition in the dentate gyrus-CA3 hippocampal circuit impacts the formation and maintenance of context-specific ensembles in CA1 and the anterior cingulate cortex. However not all the claims of this manuscript are fully supported by the data.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Memories encoded in the dentate gyrus (DG) ‒ CA3 circuit of the hippocampus are routed from CA1 to anterior cingulate cortex (ACC) for consolidation. Although CA1 parvalbumin inhibitory neurons (PV INs) orchestrate hippocampal-cortical communication, we know less about CA3 PV INs or DG ‒ CA3 principal neuron ‒ IN circuit mechanisms that contribute to evolution of hippocampal-cortical ensembles during memory consolidation. Using viral genetics to selectively mimic and boost an endogenous learning-dependent circuit mechanism, DG cell recruitment of CA3 PV INs and feed-forward inhibition (FFI) in CA3, in combination with longitudinal in vivo calcium imaging, we demonstrate that FFI facilitates formation and maintenance of context-associated neuronal ensembles in CA1. Increasing FFI in DG ‒ CA3 promoted context specificity of neuronal ensembles in ACC over time and enhanced long-term contextual fear memory. In vivo LFP recordings in mice with increased FFI in DG ‒ CA3 identified enhanced CA1 sharp-wave ripple ‒ ACC spindle coupling as a potential network mechanism facilitating memory consolidation. Our findings illuminate how FFI in DG ‒ CA3 dictates evolution of ensemble properties in CA1 and ACC during memory consolidation and suggest a teacher-like function for hippocampal CA1 in stabilization and re-organization of cortical representations.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
This ms targets an interesting question, whether changes of feedforward inhibition at the DG-CA3 synapses regulate the representational capabilities of contextual fear memory at CA1 and the anterior cingulate cortex (ACC). The paper exploits a recent tool developed by the group (viral-mediated shRNA interference of Ablim3 in DG), to enhance PV+ mediated inhibition of CA3 pyramidal cells by increasing both their recruitment by DG cells and their number of contacts over postsynaptic cells. Using micro-endoscopic imaging of mice experiencing contextual fear conditioning, the authors nicely evaluate the effect of feedforward inhibitory control of CA3 outputs in the formation, stabilization and specificity of contextual fear memory representations in the CA1 and ACC. Data is relevant to …
Author Response:
Reviewer #1 (Public Review):
This ms targets an interesting question, whether changes of feedforward inhibition at the DG-CA3 synapses regulate the representational capabilities of contextual fear memory at CA1 and the anterior cingulate cortex (ACC). The paper exploits a recent tool developed by the group (viral-mediated shRNA interference of Ablim3 in DG), to enhance PV+ mediated inhibition of CA3 pyramidal cells by increasing both their recruitment by DG cells and their number of contacts over postsynaptic cells. Using micro-endoscopic imaging of mice experiencing contextual fear conditioning, the authors nicely evaluate the effect of feedforward inhibitory control of CA3 outputs in the formation, stabilization and specificity of contextual fear memory representations in the CA1 and ACC. Data is relevant to understand how specific microcircuit motifs can influence representational dynamics in downstream regions. I have some methodological comments and recommendations for authors to improve their presentation and to exclude potential confounding factors.
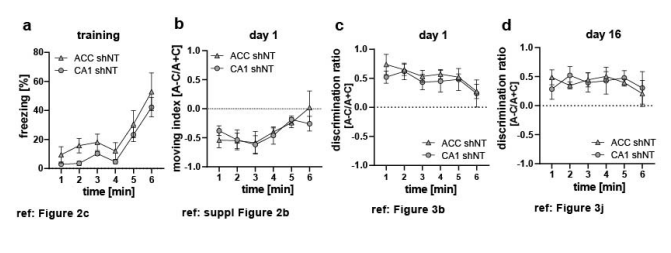
1- Since imaging is performed in CA1 and ACC separately, the study design entails 4 groups: shNT vs shRNA which is the main experimental manipulation, plus CA1 vs ACC. While data is in general carefully presented, some analysis may require additional validation to discard whether some regional effects caused by manipulation may actually reflect group differences. This is important because there may be some differences between ACC and CA1 groups in some behavioral readout (e.g. Fig.2c; Fig.S2b) which may actually explains different effect of manipulation. Formal comparisons of behavior in ACC and CA1 shNT groups may be required to discard this effect.
We compared behavior data in the control groups across brain region to test if our calcium imaging findings are driven by differences in groups rather than virus manipulation. We did not find a significant difference for any of the data sets (see figure legend Rebuttal Figure 1 a-d for details). In general, we tried to avoid presenting the same (or part of the same) dataset in multiple figures. An alternative would be to plot all 4 groups in 1 graph and test as such but that would decrease readability in our opinion. Therefore, we are happy to provide the additional graphs and analysis but prefer not to include them in the main manuscript. (Rebuttal Figure 1a-d).
<a href= “https://cdn.elifesciences.org/public-review-media/public-reviews/Y4mLzrg.png">
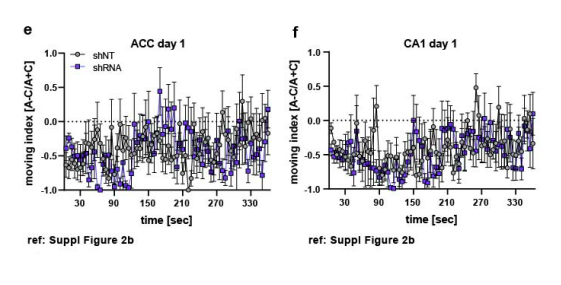
2- Differences of activity level (calcium rate) are examined using bins of 5 seconds for a total of 360 sec of exploratory activity. To discard motility effects an analysis is implemented using 1 sec bins. Thus, the two data samples are not commensurate. Also, an ANOVA on calcium rate is applied over uneven multiple comparisons to account for statistical effects of region x time or context x time. This is relevant for fig.1g vs 1i and Fig.S2j,l and may require correction.
We assume you mean “1 minute” and not “1 sec” here. We presented the two datasets (calcium event rate) and moving index indeed using different time bins (5 sec and 1 minute respectively). It is true that a difference in binning and therefore different sample size in one factor (time) could affect the result of the ANOVA. Rebuttal Figure 1 e-f shows the behavior comparison made in Suppl.Figure 2b in the original manuscript with a 5 second bin. A 2-Way ANOVA with repeated measurements reveals no main virus effect [Two-way repeated measures ANOVA, ACC (e): virus x time effect 0.0113; virus main effect N.S., time main effect N.S., n=5 per group; CA1 (f): virus x time effect N.S.; virus main effect N.S., time main effect N.S., n=5 shNT, n=6 shRNA]. In ACC, we find a significant interaction effect but a posthoc Sidak test did not reveal a difference between virus groups at any time point. This confirms our previous findings that differences in movement do not seem to drive the differences between virus groups.
<a href= “https://cdn.elifesciences.org/public-review-media/public-reviews/0x0xqqw.png">
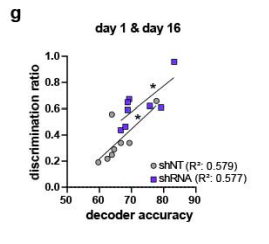
3- Fig.3 nicely show accurate context classification based on calcium activity from A&C contexts neurons using support-vector machine. The authors report very interesting representational effects for shNT vs shRNA manipulations. Is prediction accuracy of the SVM classifier correlated with behavioral discrimination? That would reinforce conclusions.
Thank you for raising this very interesting point and indeed, we found a positive correlation between the discrimination ratio and the accuracy of the SVM classifier (Pearson’s r, shNT: R2 = 0.5794, p= 0.0282, n=4; shRNA: R2= 0.5771, p= 0.0288 , n=4. We added these data in Figure 4 (Figure 4c) and in Rebuttal Figure 1g.
<a href= “https://cdn.elifesciences.org/public-review-media/public-reviews/J8mZhfJ.png">
Regarding conclusions and physiological relevance, the authors may need to discuss why enhanced feedforward inhibition at DG-CA3 synapses is not naturally established given the beneficial effect in context discrimination.
We apologize that we did not make that aspect of our manipulation clearer in our discussion. We edited the introduction and discussion (LL 65, LL 365) to clearly convey that FFI in DG-CA3 is naturally temporarily increased following learning (Ruediger 2011, Ruediger 2012, Guo et al 2018).
Reviewer #3 (Public Review):
In this study, Twarkowski et al. aim to understand the role of a specific circuit motif, dentate gyrus (DG) to CA3 feed-forward inhibition (FFI), for memory encoding and consolidation. FFI is a ubiquitous circuit motif in the brain. As a result, providing insights on its function is an interesting and a potentially very impactful contribution to neuroscience.
To tackle this issue, the authors describe how increasing DG-CA3 FFI impacts the ensemble activity in hippocampal area CA1 and the anterior cingulate cortex (ACC) in mice undergoing a contextual fear conditioning paradigm. To selectively increase FFI onto CA3 neurons, the study uses a molecular tool (downregulation of Ablim3 using virally mediated expression of shRNA), which has been developed by the same group (Guo et al, 2018, Nature Medicine). The impact of this manipulation is assessed via chronic in vivo one-photon Ca2+ imaging of dorsal CA1 and ACC neurons on the day of fear conditioning, one day after (recent recall), and 16 days after (remote recall) the fear conditioning. During and after fear conditioning, the results show in both experimental groups (shRNA and control) various population activity changes in both CA1 and ACC. Furthermore, the study finds improved context discrimination in the shRNA group only at the remote recall timepoint. The authors' conclusion is that increasing FFI enhances the formation of learning-specific ensembles, first in CA1 and later in ACC, which is associated with an improved memory recall. The experiments presented here were very technically challenging and produced a comprehensive and valuable dataset describing the parallel ensemble activity changes in CA1 and ACC after fear conditioning, with or without increasing DG-CA3 FFI. However, a causal relationship between the manipulation of DG-CA3 FFI, the network activity changes in CA1 and ACC, and the behavioral improvement is, in my opinion, not fully demonstrated. This is for a couple of reasons:
- The magnitude of the effect of the shRNA manipulation on the immediate downstream area CA3 remains unclear. Therefore, the findings in the downstream areas CA1 or even ACC (which is at least three synapses removed from CA3) are, in my opinion, difficult to interpret. This uncertainty includes (1) the extent of the virus injection in the dentate gyrus and the extent of subsequent changes in CA3, and (2) the effect of the manipulation on CA3 pyramidal cell activity in vivo. The original paper (Guo et al, 2018) uses in vitro voltage-clamp recordings to record EPSCs/IPSCs in CA3, but does not exclude possible compensatory changes in vivo, e.g., in the excitability of CA3 neurons, which could result from increasing FFI chronically over a few weeks. The data in Figures 1f and g seems to suggest that there are baseline activity changes in CA1, which might be caused by changes in the upstream CA3 network activity. Along the same lines, I am unsure how to interpret the comparisons between CA1 and ACC in Figure 1; within brain region comparisons are more relevant and should be shown instead.
This is a great point and was raised by all reviewers. We acknowledge the weakness of this comparison, apologize for this misstep in our analysis and have accordingly, removed this dataset from our manuscript. Instead, we performed new experiments using in vivo electrophysiology to allow for cross-region comparison of LFPs in CA1 and ACC within the same animal. We removed data from Figure 1 e-i and added new, simultaneous electrophysiological LFP recordings (Figure 5 and supplementary Figure 4 in revised manuscript).
We found an increased number of CA1 ripples that are coupled with ACC spindles (“coupled ripples”) in shRNA mice compared to control mice prior to a learning event (Figure 5c, two-tailed unpaired student’s t-test with Welch’s correction, p=0.0499, n=5) with no difference in time spend in slow-wave sleep (SWS) (supplementary Figure 4a) or total numbers of spindles or ripples (supplementary Figure 4b-c). Control mice show a learning-dependent increase in coupled ripples (Figure 5f, two-tailed paired student’s t-test, p=0.019, n=5) to a similar level as seen in shRNA mice prior to learning. No further increase is seen in shRNA mice indicating a saturation of circuit changes that cannot be further amplified following learning.
- Several parameters are used in this study to describe the network activity in CA1 and ACC. These include the number of correlated neuron pairs, the number of neurons active in both the training context and a neutral context (so-called A-C neurons), or the event rate observed in these A-C neurons. Most of the activity changes observed do not appear specific to the shRNA group and occur also under control condition, suggesting that they are not caused by an increase in DG-CA3 FFI. It would be helpful to clarify the sequence, how increasing FFI onto CA3 is hypothesized to cause the changes in CA1 or even ACC.
We apologize for failing to make this clearer. Prior work has shown that learning increases FFI in DG-CA3 and downregulates Ablim3 in DG (Ruediger 2011, 2012, Guo et al 2018). Therefore, it is not surprising that we observe similar changes in the control (shNT) group as shRNA group.
From previous work we know that shNT mice show increased DG-CA3 FFI following learning (training day) for approximately 24 hours (Guo et al, 2018). Thus, our manipulation allows us to mimic and boost a naturally occurring learning-induced synaptic modification in an inhibitory microcircuit in DGCA3 and examine the impact on network mechanisms underlying systems consolidation. Importantly, enhanced feedforward inhibition at the DG-CA3 synapses is naturally established for several hours following a spatial learning event (see Ruediger et al, 2011, Guo et al, 2018). Leveraging a molecular tool to enhance FFI prior to learning, we were able to reveal that DG-CA3 FFI plays a role in tuning the circuit towards cross-regional long-term storage of precise neuronal representations. (see also edits in text, LL 365).
-

Evaluation Summary:
This paper will be of interest to scientists across systems neuroscience or to those interested in how one component of a neural circuit contributes to downstream functions longitudinally. This study investigates how increasing feed forward inhibition in the dentate gyrus-CA3 hippocampal circuit impacts the formation and maintenance of context-specific ensembles in CA1 and the anterior cingulate cortex. However not all the claims of this manuscript are fully supported by the data.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This ms targets an interesting question, whether changes of feedforward inhibition at the DG-CA3 synapses regulate the representational capabilities of contextual fear memory at CA1 and the anterior cingulate cortex (ACC). The paper exploits a recent tool developed by the group (viral-mediated shRNA interference of Ablim3 in DG), to enhance PV+ mediated inhibition of CA3 pyramidal cells by increasing both their recruitment by DG cells and their number of contacts over postsynaptic cells. Using micro-endoscopic imaging of mice experiencing contextual fear conditioning, the authors nicely evaluate the effect of feedforward inhibitory control of CA3 outputs in the formation, stabilization and specificity of contextual fear memory representations in the CA1 and ACC. Data is relevant to understand how specific …
Reviewer #1 (Public Review):
This ms targets an interesting question, whether changes of feedforward inhibition at the DG-CA3 synapses regulate the representational capabilities of contextual fear memory at CA1 and the anterior cingulate cortex (ACC). The paper exploits a recent tool developed by the group (viral-mediated shRNA interference of Ablim3 in DG), to enhance PV+ mediated inhibition of CA3 pyramidal cells by increasing both their recruitment by DG cells and their number of contacts over postsynaptic cells. Using micro-endoscopic imaging of mice experiencing contextual fear conditioning, the authors nicely evaluate the effect of feedforward inhibitory control of CA3 outputs in the formation, stabilization and specificity of contextual fear memory representations in the CA1 and ACC. Data is relevant to understand how specific microcircuit motifs can influence representational dynamics in downstream regions.
I have some methodological comments and recommendations for authors to improve their presentation and to exclude potential confounding factors.
Since imaging is performed in CA1 and ACC separately, the study design entails 4 groups: shNT vs shRNA which is the main experimental manipulation, plus CA1 vs ACC. While data is in general carefully presented, some analysis may require additional validation to discard whether some regional effects caused by manipulation may actually reflect group differences. This is important because there may be some differences between ACC and CA1 groups in some behavioral readout (e.g. Fig.2c; Fig.S2b) which may actually explains different effect of manipulation. Formal comparisons of behavior in ACC and CA1 shNT groups may be required to discard this effect.
Differences of activity level (calcium rate) are examined using bins of 5 seconds for a total of 360 sec of exploratory activity. To discard motility effects an analysis is implemented using 1 sec bins. Thus, the two data samples are not commensurate. Also, an ANOVA on calcium rate is applied over uneven multiple comparisons to account for statistical effects of region x time or context x time. This is relevant for fig.1g vs 1i and Fig.S2j,l and may require correction.
Figure 3 nicely show accurate context classification based on calcium activity from A&C contexts neurons using support-vector machine. The authors report very interesting representational effects for shNT vs shRNA manipulations. Is prediction accuracy of the SVM classifier correlated with behavioral discrimination? That would reinforce conclusions.
Regarding conclusions and physiological relevance, the authors may need to discuss why enhanced feedforward inhibition at DG-CA3 synapses is not naturally established given the beneficial effect in context discrimination.
-

Reviewer #2 (Public Review):
In this manuscript, Twarkowski and colleagues use a previously published lentiviral technique to boost dentate gyrus (DG) mossy fiber recruitment of CA3 parvalbumin+ inhibitory neurons, thus increasing feedforward inhibition in the DG - CA3 circuit. Using this technique, the authors test the impact of increasing feedforward inhibition (FFI) in this circuit on the formation and maintenance of context-associated neuronal ensembles in CA1 and the downstream anterior cingulate cortex (ACC). The authors begin by replicating their previous work showing that increasing FFI in CA3 enhances context-specific freezing behaviors at remote timepoints and assess the correlation structure of CA1 and ACC populations during recent and remote recall. The authors claim that FFI "facilitates formation and maintenance of …
Reviewer #2 (Public Review):
In this manuscript, Twarkowski and colleagues use a previously published lentiviral technique to boost dentate gyrus (DG) mossy fiber recruitment of CA3 parvalbumin+ inhibitory neurons, thus increasing feedforward inhibition in the DG - CA3 circuit. Using this technique, the authors test the impact of increasing feedforward inhibition (FFI) in this circuit on the formation and maintenance of context-associated neuronal ensembles in CA1 and the downstream anterior cingulate cortex (ACC). The authors begin by replicating their previous work showing that increasing FFI in CA3 enhances context-specific freezing behaviors at remote timepoints and assess the correlation structure of CA1 and ACC populations during recent and remote recall. The authors claim that FFI "facilitates formation and maintenance of context-associated neuronal ensembles in CA1" but most of the direct tests between groups are not performed, and it's unclear how increasing correlation structure within a recall session relates to the maintenance of ensembles and memory performance. The premise of interrogating a circuit on this synapse-specific scale without direct interference/stimulation of broader elements of that circuit is an exciting and important direction for systems neuroscience. Overall, this manuscript is written and organized in an intuitive way, however, many of the findings have been previously published by this group and other claims are not yet supported by the data and need to be amended or clarified.
-

Reviewer #3 (Public Review):
In this study, Twarkowski et al. aim to understand the role of a specific circuit motif, dentate gyrus (DG) to CA3 feed-forward inhibition (FFI), for memory encoding and consolidation. FFI is a ubiquitous circuit motif in the brain. As a result, providing insights on its function is an interesting and a potentially very impactful contribution to neuroscience.
To tackle this issue, the authors describe how increasing DG-CA3 FFI impacts the ensemble activity in hippocampal area CA1 and the anterior cingulate cortex (ACC) in mice undergoing a contextual fear conditioning paradigm. To selectively increase FFI onto CA3 neurons, the study uses a molecular tool (downregulation of Ablim3 using virally mediated expression of shRNA), which has been developed by the same group (Guo et al, 2018, Nature Medicine). The …
Reviewer #3 (Public Review):
In this study, Twarkowski et al. aim to understand the role of a specific circuit motif, dentate gyrus (DG) to CA3 feed-forward inhibition (FFI), for memory encoding and consolidation. FFI is a ubiquitous circuit motif in the brain. As a result, providing insights on its function is an interesting and a potentially very impactful contribution to neuroscience.
To tackle this issue, the authors describe how increasing DG-CA3 FFI impacts the ensemble activity in hippocampal area CA1 and the anterior cingulate cortex (ACC) in mice undergoing a contextual fear conditioning paradigm. To selectively increase FFI onto CA3 neurons, the study uses a molecular tool (downregulation of Ablim3 using virally mediated expression of shRNA), which has been developed by the same group (Guo et al, 2018, Nature Medicine). The impact of this manipulation is assessed via chronic in vivo one-photon Ca2+ imaging of dorsal CA1 and ACC neurons on the day of fear conditioning, one day after (recent recall), and 16 days after (remote recall) the fear conditioning. During and after fear conditioning, the results show in both experimental groups (shRNA and control) various population activity changes in both CA1 and ACC. Furthermore, the study finds improved context discrimination in the shRNA group only at the remote recall timepoint. The authors' conclusion is that increasing FFI enhances the formation of learning-specific ensembles, first in CA1 and later in ACC, which is associated with an improved memory recall. The experiments presented here were very technically challenging and produced a comprehensive and valuable dataset describing the parallel ensemble activity changes in CA1 and ACC after fear conditioning, with or without increasing DG-CA3 FFI. However, a causal relationship between the manipulation of DG-CA3 FFI, the network activity changes in CA1 and ACC, and the behavioral improvement is, in my opinion, not fully demonstrated. This is for a couple of reasons:
The magnitude of the effect of the shRNA manipulation on the immediate downstream area CA3 remains unclear. Therefore, the findings in the downstream areas CA1 or even ACC (which is at least three synapses removed from CA3) are, in my opinion, difficult to interpret. This uncertainty includes (1) the extent of the virus injection in the dentate gyrus and the extent of subsequent changes in CA3, and (2) the effect of the manipulation on CA3 pyramidal cell activity in vivo. The original paper (Guo et al, 2018) uses in vitro voltage-clamp recordings to record EPSCs/IPSCs in CA3, but does not exclude possible compensatory changes in vivo, e.g., in the excitability of CA3 neurons, which could result from increasing FFI chronically over a few weeks. The data in Figures 1f and g seems to suggest that there are baseline activity changes in CA1, which might be caused by changes in the upstream CA3 network activity. Along the same lines, I am unsure how to interpret the comparisons between CA1 and ACC in Figure 1; within brain region comparisons are more relevant and should be shown instead.
Several parameters are used in this study to describe the network activity in CA1 and ACC. These include the number of correlated neuron pairs, the number of neurons active in both the training context and a neutral context (so-called A-C neurons), or the event rate observed in these A-C neurons. Most of the activity changes observed do not appear specific to the shRNA group and occur also under control condition, suggesting that they are not caused by an increase in DG-CA3 FFI. It would be helpful to clarify the sequence, how increasing FFI onto CA3 is hypothesized to cause the changes in CA1 or even ACC.
-
