Homotopic contralesional excitation suppresses spontaneous circuit repair and global network reconnections following ischemic stroke
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Bice et al. present new work using an optogenetics-based stimulation to test how this affects stroke recovery in mice. Namely, can they determine if contralateral stimulation of S1 would enhance or hinder recovery after a stroke? They found that stimulation of the cortex contralateral to the site of stroke impairs recovery from this stroke, and impairs the brain mapping and the connectivity that normally emerges in recovery from stroke. This unexpected finding in a mouse model relates to clinical literature on the role of the contralateral cortex in recovery.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Understanding circuit-level manipulations that affect the brain’s capacity for plasticity will inform the design of targeted interventions that enhance recovery after stroke. Following stroke, increased contralesional activity (e.g. use of the unaffected limb) can negatively influence recovery, but it is unknown which specific neural connections exert this influence, and to what extent increased contralesional activity affects systems- and molecular-level biomarkers of recovery. Here, we combine optogenetic photostimulation with optical intrinsic signal imaging to examine how contralesional excitatory activity affects cortical remodeling after stroke in mice. Following photothrombosis of left primary somatosensory forepaw (S1FP) cortex, mice either recovered spontaneously or received chronic optogenetic excitation of right S1FP over the course of 4 weeks. Contralesional excitation suppressed perilesional S1FP remapping and was associated with abnormal patterns of stimulus-evoked activity in the unaffected limb. This maneuver also prevented the restoration of resting-state functional connectivity (RSFC) within the S1FP network, RSFC in several networks functionally distinct from somatomotor regions, and resulted in persistent limb-use asymmetry. In stimulated mice, perilesional tissue exhibited transcriptional changes in several genes relevant for recovery. Our results suggest that contralesional excitation impedes local and global circuit reconnection through suppression of cortical activity and several neuroplasticity-related genes after stroke, and highlight the importance of site selection for targeted therapeutic interventions after focal ischemia.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
Bice et al. present new work using an optogenetics-based stimulation to test how this affects stroke recovery in mice. Namely, can they determine if contralateral stimulation of S1 would enhance or hinder recovery after a stroke? The study provides interesting evidence that this stimulation may be harmful, and not helpful. They found that contralesional optogenetic-based excitation suppressed perilesional S1FP remapping, and this caused abnormal patterns of evoked activity in the unaffected limb. They applied a network analysis framework and found that stimulation prevented the restoration of resting-state functional connectivity within the S1FP network, and resulted in limb-use asymmetry in the mice. I think it's an important finding. My suggestions for improvement revolve around …
Author Response
Reviewer #1 (Public Review):
Bice et al. present new work using an optogenetics-based stimulation to test how this affects stroke recovery in mice. Namely, can they determine if contralateral stimulation of S1 would enhance or hinder recovery after a stroke? The study provides interesting evidence that this stimulation may be harmful, and not helpful. They found that contralesional optogenetic-based excitation suppressed perilesional S1FP remapping, and this caused abnormal patterns of evoked activity in the unaffected limb. They applied a network analysis framework and found that stimulation prevented the restoration of resting-state functional connectivity within the S1FP network, and resulted in limb-use asymmetry in the mice. I think it's an important finding. My suggestions for improvement revolve around quantitative analysis of the behavior, but the experiments are otherwise convincing and important.
Thank you for the positive feedback regarding our work.
Other comments - Data and paper presentation:
- Figure 1A is misleading; it appears as if optogenetic stimulation is constant (which indeed would be detrimental to the tissue). Also, the atlas map overlaps color-wise with conditions; at a glance it looks like the posterior cortex might be stimulated; consider making greyscale?
We have updated Figure 1A to address these concerns.
Reviewer #2 (Public Review):
These studies test the effect of stimulation of the contralateral somatosensory cortex on recovery, evoked responses, functional interconnectivity and gene expression in a somatosensory cortex stroke. Using transgenic mice with ChR2 in excitatory neurons, these neurons are stimulated in somatosensory cortex from days 1 after stroke to 4 weeks. This stimulation is fairly brief: 3min/day. Mice then received behavioral analysis, electrical forepaw stimulation and optical intrinsic signal mapping, and resting state MRI. The core finding is that this ChR2 stimulation of excitatory neurons in contralateral somatosensory cortex impairs recovery, evoked activity and interconnectivity of contralateral (to the stimulation, ipsilateral to the stroke) cortex in this localized stroke model. This is a surprising result, and resonates with some clinical findings, and a robust clinical discussion, on the role of the contralateral cortex in recovery. This manuscript addresses several important topics. The issue of brain stimulation and alterations in brain activity that the studies explore are also part of human brain stimulation protocols, and pre-clinical studies. The finding that contralateral stimulation inhibits recovery and functional circuit remapping is an important one. The rsMRI analysis is sophisticated.
Thank you for the supportive comments regarding our manuscript
Concerns:
- The gene expression data is to be expected. Stimulation of the brain in almost any context alters the expression of genes.
We agree with the reviewer that stimulation of the brain is expected to broadly alter gene expression. However, in this set of studies, we examined a subset of genes that are of particular interest in neuroplasticity, and compared expression in ipsi-lesional vs. contra-lesional cortex in the presence or absence of contralesional stimulation during the post stroke recovery period. Genes like Arc, for example, have been shown by our group to be necessary for perilesional plasticity and recovery (Kraft, et al., Science Translational Medicine, 2018). The finding that validated plasticity genes are suppressed by contralesional stimulation is consistent with the central finding that contralesional stimulation suppresses the recovery of normal patterns of brain organization and activity. Importantly, there were also genes associated with spontaneous recovery that were unaltered or increased by contra-lesional brain stimulation. While these data do not provide causal associations, they may prove to be useful for developing hypotheses regarding molecular mechanisms involved in spontaneous brain repair for future studies.
In light of the reviewer’s comment, we have altered text throughout to not focus on specific directionality of transcripts. Instead, we indicate that relevant transcript changes are those that are altered in association with spontaneous recovery, and which are altered in the opposite direction with contralesional brain stimulation.
Minor points.
- Was the behavior and the functional imaging done while the brain was being stimulated?
We have updated the methods (page 17) to clarify that the only experiments during which the photostimulus occurred during neuroimaging are reported in new Figure 6, and to clarify that photostimulation did not occur during the behavioral tests of asymmetry.
- It would be useful to understand what is being stimulated. The stimulation method is not described. Is an entire cortical width of tissue stimulated, and this is what is feeding back onto the contralateral cortex? Or is this stimulation mostly affecting excitatory (CaMKII+) cells in upper or lower layers? This will be important to be able to compare to the Chen et al study that gave rise to the stimulation approach here. This gets to the issue of the circuitry that is important in recovery, or in inhibiting recovery. One might answer this question by doing the stimulation and staining tissue for immediate early gene activation, to see the circuits with evoked activity. Also, the techniques used in this study could be applied with OIS or rsMRI during stimulation, to determine the circuits that are activated.
We have clarified the stimulation protocol in response to Essential point 2.2. Due to light scattering and appreciable attenuation of 473nm in brain tissue, only ~1% of photons penetrate to a depth of 600 microns. Experimentally, this provides superficial-layer specificity to Layer 2/3 Camk2a cells (https://doi.org/10.1016/j.neuron.2011.06.004)
To answer the question of what circuits are affecting recovery, we performed 2 sets of additional experiments – Experiment 1: OISI during photostimulation before and after photothrombosis, and Experiment 2: tissue staining for IEG expression (cFOS). We describe each below:
Experiment 1 New results are included from 16 Camk2a-ChR2 mice (Results, page 10-11; Methods, page 18) and reported as new Figure 6. Similar to the previously reported experiments, all mice were subject to photothrombosis of left S1FP, half of which received interventional optogenetic photostimulation beginning 1 day after photothrombosis (+Stim) while the other half recovered spontaneously (-Stim). To visualize in real time whether contralesional photostimulation differentially affected global cortical activity in these 2 groups, concurrent awake OISI during acute contralesional photostimulation was performed in +Stim and –Stim groups before, 1, and 4 weeks after photothrombosis. At baseline, all mice exhibited focal increases in right S1FP activity during photostimulation that spread to contralateral (left) S1FP and other motor regions approximately 8-10 seconds after stimulus onset. While activity increases within the targeted circuit, subtle inhibition of cortical activity can also be observed in surrounding non-targeted cortices. Thus, activity both increases and decreases in different cortical regions during and after optogenetic stimulation of the right S1FP circuit. Of note, regions that are inhibited by S1FP stimulation show more pronounced decreases in activity in +Stim mice at 1 and 4 weeks compared to baseline and were significantly larger in +Stim mice compared to –Stim mice. We conclude that focal stimulation of contralesional cortex results in significant, widespread inhibitory influences that extend well beyond the targeted circuit.
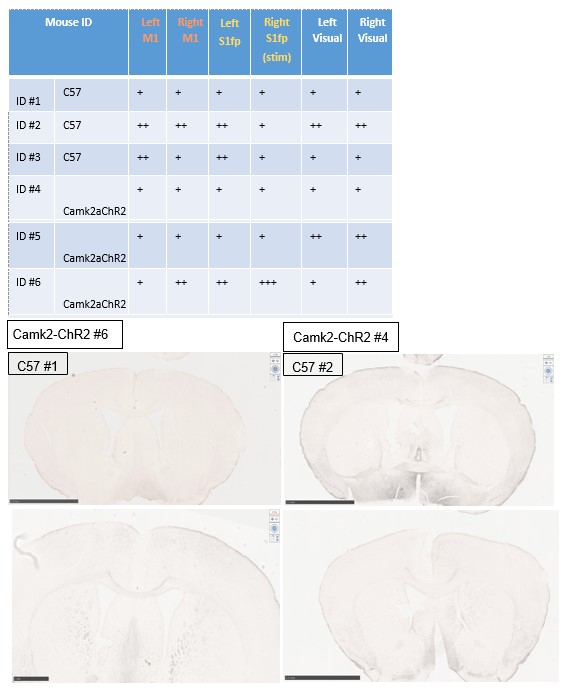
Experiment 2 For experiment 2, we hypothesized that IEG expression would increase in photostimulated regions, cortical regions functionally connected to targeted areas, and potentially deeper brain regions. For the IEG experiments, healthy ChR2 naïve animals (C57 mice) or CamK2a-ChR2 mice were acclimated to the head-restraint apparatus described in the manuscript used for photostimulation treatment. Once trained, awake mice were subject to the same photostimulus protocol as described in the manuscript applied to forepaw somatosensory cortex in the right hemisphere. After stimulation, mice were sacrificed, perfused, and brains were harvested for tissue slicing and immunostaining for cFOS. Tissue slices containing right and left primary forepaw somatosensory cortex and primary and secondary motor cortices (+0.5mm A/P) or visual cortex (-2.8mm A/P) were examined for cFOS staining and compared across groups.
Below is a summary table of our findings, and representative tissue slices. While c-FOS IHC was successful, results are not consistent with expectations from the mouse strains used. Only 1 ChR2+ mouse exhibited staining patterns consistent with local S1FP photostimulation, while expression in ChR2- mice was more variable, and in some instances exhibits higher expression in targeted circuits compared to ChR2+ mice. It is possible that awake behaving mice already exhibit high activity in sensorimotor cortex at rest, which might obscure changes specific to optogenetic photostimulation. Regardless, because the tissue staining experiments were inconclusive in healthy animals, we did not proceed with further experiments in the stroke groups, and do not report these findings in the manuscript.
- Also, it is possible that contralateral stimulation is impairing recovery, not through an effect on the contralateral cortex (the site of the stroke), but on descending projections, or theoretically even through evoking activity or subclinical movement of the contralateral limb (ipsilateral to the stroke). By more carefully mapping the distribution of the activity of the stimulated brain region, and what exactly is being stimulated, these issues can be explored.
The reviewer raises an excellent point. We have added to the “Limitations and Future work” section of the Discussion on pages 15-16
-

Evaluation Summary:
Bice et al. present new work using an optogenetics-based stimulation to test how this affects stroke recovery in mice. Namely, can they determine if contralateral stimulation of S1 would enhance or hinder recovery after a stroke? They found that stimulation of the cortex contralateral to the site of stroke impairs recovery from this stroke, and impairs the brain mapping and the connectivity that normally emerges in recovery from stroke. This unexpected finding in a mouse model relates to clinical literature on the role of the contralateral cortex in recovery.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
Bice et al. present new work using an optogenetics-based stimulation to test how this affects stroke recovery in mice. Namely, can they determine if contralateral stimulation of S1 would enhance or hinder recovery after a stroke? The study provides interesting evidence that this stimulation may be harmful, and not helpful. They found that contralesional optogenetic-based excitation suppressed perilesional S1FP remapping, and this caused abnormal patterns of evoked activity in the unaffected limb. They applied a network analysis framework and found that stimulation prevented the restoration of resting-state functional connectivity within the S1FP network, and resulted in limb-use asymmetry in the mice. I think it's an important finding. My suggestions for improvement revolve around quantitative analysis of …
Reviewer #1 (Public Review):
Bice et al. present new work using an optogenetics-based stimulation to test how this affects stroke recovery in mice. Namely, can they determine if contralateral stimulation of S1 would enhance or hinder recovery after a stroke? The study provides interesting evidence that this stimulation may be harmful, and not helpful. They found that contralesional optogenetic-based excitation suppressed perilesional S1FP remapping, and this caused abnormal patterns of evoked activity in the unaffected limb. They applied a network analysis framework and found that stimulation prevented the restoration of resting-state functional connectivity within the S1FP network, and resulted in limb-use asymmetry in the mice. I think it's an important finding. My suggestions for improvement revolve around quantitative analysis of the behavior, but the experiments are otherwise convincing and important.
However, the behavioral readout is not well documented and, to me, is a key readout for the main claims in the paper. For example, in the methods; "The laser power ranged between 0.2mW - 1mW and was set to a level just below that which elicited overt behavioral output (e.g. forepaw or whisker motor movements in sync with stimuli)." for example, how is this measured? By eye? Quantitatively with tracking approaches? Also, the cylinder test I have the same concern - it notes there is only 1 blinded scorer, but how consistent is this person? Why not use a machine vision approach (if the videos are saved, I strongly suggest this is quantified differently). If not, they should add this to the limitations section in discussion.
Other comments - Data and paper presentation:
- Figure 1A is misleading; it appears as if optogenetic stimulation is constant (which indeed would be detrimental to the tissue). Also, the atlas map overlaps color-wise with conditions; at a glance it looks like the posterior cortex might be stimulated; consider making greyscale?
-

Reviewer #2 (Public Review):
These studies test the effect of stimulation of the contralateral somatosensory cortex on recovery, evoked responses, functional interconnectivity and gene expression in a somatosensory cortex stroke. Using transgenic mice with ChR2 in excitatory neurons, these neurons are stimulated in somatosensory cortex from days 1 after stroke to 4 weeks. This stimulation is fairly brief: 3min/day. Mice then received behavioral analysis, electrical forepaw stimulation and optical intrinsic signal mapping, and resting state MRI. The core finding is that this ChR2 stimulation of excitatory neurons in contralateral somatosensory cortex impairs recovery, evoked activity and interconnectivity of contralateral (to the stimulation, ipsilateral to the stroke) cortex in this localized stroke model. This is a surprising result, …
Reviewer #2 (Public Review):
These studies test the effect of stimulation of the contralateral somatosensory cortex on recovery, evoked responses, functional interconnectivity and gene expression in a somatosensory cortex stroke. Using transgenic mice with ChR2 in excitatory neurons, these neurons are stimulated in somatosensory cortex from days 1 after stroke to 4 weeks. This stimulation is fairly brief: 3min/day. Mice then received behavioral analysis, electrical forepaw stimulation and optical intrinsic signal mapping, and resting state MRI. The core finding is that this ChR2 stimulation of excitatory neurons in contralateral somatosensory cortex impairs recovery, evoked activity and interconnectivity of contralateral (to the stimulation, ipsilateral to the stroke) cortex in this localized stroke model. This is a surprising result, and resonates with some clinical findings, and a robust clinical discussion, on the role of the contralateral cortex in recovery.
This manuscript addresses several important topics. The issue of brain stimulation and alterations in brain activity that the studies explore are also part of human brain stimulation protocols, and pre-clinical studies. The finding that contralateral stimulation inhibits recovery and functional circuit remapping is an important one. The rsMRI analysis is sophisticated.
Concerns:
1. The studies in the manuscript utilize brain stimulation, and use it as a goal of modeling behavioral limb use. In particular, the optogenetic stimulation protocol of primary somatosensory forelimb cortex is said to mimic overuse of the limb. It is not clear how stimulation of a subset of neurons in somatosensory cortex mimics limb overuse. Further, this stimulation is, in totality, very brief. Limb overuse does not appear to align with this brain stimulation protocol.
2. The parameters of the stimulation are set and not altered in these studies. They were chosen based on Cheng et al (ref 25). This is interesting because in this publication, the optical stimulation parameters, delivered into peri-stroke cortex, produced recovery. Would different stimulation parameters have a different outcome on recovery or functional connectivity? Or is this contralateral stimulation site the main determinant of the negative effect on these two? The stimulation setup is very different in this Cheng et al study, apparently to the present study. However, this is not clear as the actual stimulation is not described. In Cheng et al., the stimulation was specifically to layer V pyramidal neurons with an optrode in Thy1-ChR2 mice. A cranial window is described in the methods section, but this appears to be for imaging. How were the ChR2 neurons stimulated in this present study?
3. Relative to the last issue in #2, if the stimulation was done with an implanted optrode, what was done with control: the -stim condition. Was this an implanted optrode but not activated? Or, if stimulation was done with an LCD on the skull or a window, was this done in -stim?
4. There is substantial and ever-deeper analysis of the rsMRI data (Figs. 4-6). This is all supportive of the overall core finding, that contralateral stimulation of neurons with ChR2 in this protocol impairs recovery. But each successive level of rsMRI analysis does not really add a new amount of evidence-this array of figures is not a new or independent set of data.
5. The gene expression data is to be expected. Stimulation of the brain in almost any context alters the expression of genes.
Minor points:
- Was the behavior and the functional imaging done while the brain was being stimulated?
- It would be useful to understand what is being stimulated. The stimulation method is not described. Is an entire cortical width of tissue stimulated, and this is what is feeding back onto the contralateral cortex? Or is this stimulation mostly affecting excitatory (CaMKII+) cells in upper or lower layers? This will be important to be able to compare to the Chen et al study that gave rise to the stimulation approach here. This gets to the issue of the circuitry that is important in recovery, or in inhibiting recover. One might answer this question by doing the stimulation and staining tissue for immediate early gene activation, to see the circuits with evoked activity. Also, the techniques used in this study could be applied with OIS or rsMRI during stimulation, to determine the circuits that are activated.
- Also, it is possible that contralateral stimulation is impairing recovery, not through an effect on the contralateral cortex (the site of the stroke), but on descending projections, or theoretically even through evoking activity or subclinical movement of the contralateral limb (ipsilateral to the stroke). By more carefully mapping the distribution of the activity of the stimulated brain region, and what exactly is being stimulated, these issues can be explored.
-

Reviewer #3 (Public Review):
These studies test the effect of stimulation of the contralateral somatosensory cortex on recovery, evoked responses, functional interconnectivity and gene expression in a somatosensory cortex stroke. Using transgenic mice with ChR2 in excitatory neurons, these neurons are stimulated in somatosensory cortex from days 1 after stroke to 4 weeks. This stimulation is fairly brief: 3min/day. Mice then received behavioral analysis, electrical forepaw stimulation and optical intrinsic signal mapping, and resting state MRI. The core finding is that this ChR2 stimulation of excitatory neurons in contralateral somatosensory cortex impairs recovery, evoked activity and interconnectivity of contralateral (to the stimulation, ipsilateral to the stroke) cortex in this localized stroke model. This is a surprising result, …
Reviewer #3 (Public Review):
These studies test the effect of stimulation of the contralateral somatosensory cortex on recovery, evoked responses, functional interconnectivity and gene expression in a somatosensory cortex stroke. Using transgenic mice with ChR2 in excitatory neurons, these neurons are stimulated in somatosensory cortex from days 1 after stroke to 4 weeks. This stimulation is fairly brief: 3min/day. Mice then received behavioral analysis, electrical forepaw stimulation and optical intrinsic signal mapping, and resting state MRI. The core finding is that this ChR2 stimulation of excitatory neurons in contralateral somatosensory cortex impairs recovery, evoked activity and interconnectivity of contralateral (to the stimulation, ipsilateral to the stroke) cortex in this localized stroke model. This is a surprising result, and resonates with some clinical findings, and a robust clinical discussion, on the role of the contralateral cortex in recovery.
This manuscript addresses several important topics. The issue of brain stimulation and alterations in brain activity that the studies explore are also part of human brain stimulation protocols, and pre-clinical studies. The finding that contralateral stimulation inhibits recovery and functional circuit remapping is an important one. The rsMRI analysis is sophisticated.
Concerns:
1. The studies in the manuscript utilize brain stimulation, and use it as a goal of modeling behavioral limb use. In particular, the optogenetic stimulation protocol of primary somatosensory forelimb cortex is said to mimic overuse of the limb. It is not clear how stimulation of a subset of neurons in somatosensory cortex mimics limb overuse. Further, this stimulation is, in totality, very brief. Limb overuse does not appear to align with this brain stimulation protocol.
2. The parameters of the stimulation are set and not altered in these studies. They were chosen based on Cheng et al (ref 25). This is interesting because in this publication, the optical stimulation parameters, delivered into peri-stroke cortex, produced recovery. Would different stimulation parameters have a different outcome on recovery or functional connectivity? Or is this contralateral stimulation site the main determinant of the negative effect on these two? The stimulation setup is very different in this Cheng et al study, apparently to the present study. However, this is not clear as the actual stimulation is not described. In Cheng et al., the stimulation was specifically to layer V pyramidal neurons with an optrode in Thy1-ChR2 mice. A cranial window is described in the methods section, but this appears to be for imaging. How were the ChR2 neurons stimulated in this present study?
3. Relative to the last issue in #2, if the stimulation was done with an implanted optrode, what was done with control: the -stim condition. Was this an implanted optrode but not activated? Or, if stimulation was done with an LCD on the skull or a window, was this done in -stim?
4. There is substantial and ever-deeper analysis of the rsMRI data (Figs. 4-6). This is all supportive of the overall core finding, that contralateral stimulation of neurons with ChR2 in this protocol impairs recovery. But each successive level of rsMRI analysis does not really add a new amount of evidence-this array of figures is not a new or independent set of data.
5. The gene expression data is to be expected. Stimulation of the brain in almost any context alters the expression of genes.
Minor points:
- Was the behavior and the functional imaging done while the brain was being stimulated?
- It would be useful to understand what is being stimulated. The stimulation method is not described. Is an entire cortical width of tissue stimulated, and this is what is feeding back onto the contralateral cortex? Or is this stimulation mostly affecting excitatory (CaMKII+) cells in upper or lower layers? This will be important to be able to compare to the Chen et al study that gave rise to the stimulation approach here. This gets to the issue of the circuitry that is important in recovery, or in inhibiting recover. One might answer this question by doing the stimulation and staining tissue for immediate early gene activation, to see the circuits with evoked activity. Also, the techniques used in this study could be applied with OIS or rsMRI during stimulation, to determine the circuits that are activated.
- Also, it is possible that contralateral stimulation is impairing recovery, not through an effect on the contralateral cortex (the site of the stroke), but on descending projections, or theoretically even through evoking activity or subclinical movement of the contralateral limb (ipsilateral to the stroke). By more carefully mapping the distribution of the activity of the stimulated brain region, and what exactly is being stimulated, these issues can be explored.
-