Left hemisphere dominance for bilateral kinematic encoding in the human brain
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This paper provides further evidence for hemispheric asymmetry in the cortical control of manual actions based on intracranial (ECoG) recordings in human participants. Specifically, based a linear encoding model, the authors argue that movement encoding is more bilateral in the left hemisphere than the right hemisphere. The paper is well-written and the analyses are largely appropriate for addressing the primary hypothesis, though it would be helpful to detail the variability of electrode placement across individuals (which arises for the clinical intervention being undertaken) and incorporate this variability into the statistical analysis. Given the novelty of this type of human data and the well established question being addressed, this paper will be of interest to both basic and clinical researchers in motor neuroscience.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
- @MariusPeelen's saved articles (MariusPeelen)
Abstract
Neurophysiological studies in humans and nonhuman primates have revealed movement representations in both the contralateral and ipsilateral hemispheres. Inspired by clinical observations, we ask if this bilateral representation differs for the left and right hemispheres. Electrocorticography was recorded in human participants during an instructed-delay reaching task, with movements produced with either the contralateral or ipsilateral arm. Using a cross-validated kinematic encoding model, we found stronger bilateral encoding in the left hemisphere, an effect that was present during preparation and was amplified during execution. Consistent with this asymmetry, we also observed better across-arm generalization in the left hemisphere, indicating similar neural representations for right and left arm movements. Notably, these left hemisphere electrodes were centered over premotor and parietal regions. The more extensive bilateral encoding in the left hemisphere adds a new perspective to the pervasive neuropsychological finding that the left hemisphere plays a dominant role in praxis.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
5.The reported data point to an important role of the premotor and parietal regions of the left as compared to the right hemisphere in the control of ipsilateral and contralateral limb movements. These are also the regions where the electrodes were primarily located in both subgroups of patients. I have 2 concerns in this respect. The first concern refers to the specific locus of these electrodes. For premotor cortex, the authors suggest PMd as well as PMv as potential sites for these bilateral representations. The other principal site refers to parietal cortex but this covers a large territory. It would help if more specific subregions for the parietal cortex can be indicated, if possible. Do the focal regions where electrodes were positioned refer to the superior vs inferior parietal …
Author Response:
Reviewer #1 (Public Review):
5.The reported data point to an important role of the premotor and parietal regions of the left as compared to the right hemisphere in the control of ipsilateral and contralateral limb movements. These are also the regions where the electrodes were primarily located in both subgroups of patients. I have 2 concerns in this respect. The first concern refers to the specific locus of these electrodes. For premotor cortex, the authors suggest PMd as well as PMv as potential sites for these bilateral representations. The other principal site refers to parietal cortex but this covers a large territory. It would help if more specific subregions for the parietal cortex can be indicated, if possible. Do the focal regions where electrodes were positioned refer to the superior vs inferior parietal cortex (anterior or posterior), or intra-parietal sulcus. Second, the manuscript's focus on the premotor-parietal complex emerges from the constraints imposed by accessible anatomical locations in the participants but does not preclude the existence of other cortical sites as well as subcortical regions and cerebellum for such bilateral representations. It is meaningful to clarify this and/or list this as a limitation of the current approach.
On the first issue, we have updated the manuscript to specify the subregion within the parietal cortex in which we see stronger across-arm generalization - namely, the superior parietal cortex. On the second issue, we have added text in the Discussion that reference subcortical areas shown to exhibit laterality differences in bimanual coordination, providing a more holistic picture of bimanual representations across the brain. In addition, we acknowledge that with our current patient population we are limited to regions with substantial electrode coverage, which does not include all areas of the brain.
6.The evidence for bilateral encoding during unilateral movement opens perspectives for a better understanding of the control of bimanual movements which are abundant during every day life. In the discussion, the authors refer to some imaging studies on bimanual control in order to infer whether the obtained findings may be a consequence of left hemisphere specialization for bimanual movement control, leading to speculations about the information that is being processed for each of both limb movements. Another perspective to consider is the possibility that making a movement with one limb may require postural stabilization in the trunk and contralateral body side, including a contribution from the opposite limb that is supposedly resting on the start button. Have the authors considered whether this postural mechanism could (partly) account for this bilateral encoding mechanism, in particular, because it appears more prominent during movement execution as compared to preparation. Furthermore, could the prominence of bilateral encoding during movement execution be triggered by inflow of sensory information about both limbs from the visual as well as the somatosensory systems.
Thank you for these comments. We have added a paragraph to the Discussion to address the hypothesis that some component of ipsilateral encoding may be related to postural stabilization.
In response to the final point in this comment, we agree that bilateral information during execution could be reflective of afferent inputs (somatosensory and/or visual). However, the encoding model shows that activity in premotor and parietal regions are well predicted based on kinematics during the task. While visual and somatosensory system information are likely integrated in these areas, the kinematic encoding would point to a more movement-based representation.
Reviewer #2 (Public Review):
Weaknesses:
- Although the current human ECoG data set is valuable, there is still large variability in electrode coverage across the patients (I fully acknowledge the difficulty). This makes statistical assessment a bit tricky. The potential factors of interest in the current study would be Electrode (=Region), Subject, Hemisphere, and their interactions. The tricky part is that Electrode is nested within Subject, and Subject is nested within Hemisphere. Permutation-based ANOVA used for the current paper requires proper treatment of these nested factors when making permutations (Anderson and Braak, 2003). With this regard, sufficient details about how the authors treated each factor, for instance, in each pbANOVA, are not provided in the current version of the manuscript. Similarly, the scope of statistical generalizability, whether the inference is within-sample or population-level, for the claims (e.g., statement about the hemispheric or regional difference) needs to be clarified.
We discuss at length the issue of electrode variability and have addressed this in the revised manuscript. Graphically, we have added a Supplemental Figure (S2). Statistically, we appreciate the point about the need for the analysis to address the nested structure of the data. We have redone all of the statistics, now using a permutation-based linear mixed effects model with a random effect of patient. This approach did not change any of the findings.
As to the comment about hemispheric or regional differences, the data show that both are important factors. Our hemispheric effect is characterized by stronger ipsilateral encoding in the left hemisphere and subsequently better across-arm generalization (Figures 2-4). We then examine the spatial distribution of electrodes that generalized well or poorly and found clusters in both hemispheres of electrodes that generalize poorly. In contrast, only in the left hemisphere did we find clusters of electrodes that generalize well. These electrodes were localized to PMd, PMv and superior parietal cortex (Fig 5D). In summary, we argue that activity patterns in M1 are similar in the left and right hemispheres, but there is a marked asymmetry for activity patterns over premotor and parietal cortices.
Additional contexts that would help readers interpret or understand the significance of the work: The greater amount of shared movement representation in the left hemisphere may imply the greater reliance of the left arm on the left hemisphere. This may, in turn, lead to the greater influence of the ongoing right arm motion on the left arm movement control during the bimanual coordination. Indeed, this point is addressed by the authors in the Discussion (page 15, lines 26-41). One critical piece of literature missing in this context is the work done by Yokoi, Hirashima, and Nozaki (2014). In the experiments using the bimanual reaching task, they in fact found that the learning by the left arm is to the greater degree influenced by the concurrent motion of the right arm than vice versa (Yokoi et al., J Neurosci, 2014). Together with Diedrichsen et al. (2013), this study will strengthen the authors' discussion and help readers interpret the present result of left hemisphere dominance in the context of more skillful bimanual action.
The Yokoi paper is a very important paper in revealing hemispheric asymmetries during skilled bimanual movements. However, we think it is problematic to link the hemispheric asymmetries we observe to the behavioral effects reported in the Yokoi paper (namely, that the nondominant, left arm was more strongly influenced by the kinematics of the right arm). One could hypothesize that the left hemisphere, given its representation of both arms, could be controlling both arms in some sort of direct way (and thus the action of the right arm will have an influence on left arm movement given the engagement of the same neural regions for both movements). It is also possible that the left hemisphere is receiving information about the state of both the right and left arms, and this underlies the behavioral asymmetry reported in Yokoi.
Reviewer #3 (Public Review):
In the present work, Merrick et al. analyzed ECoG recordings from patients performing out-and-back reaching movements. The authors trained a linear model to map kinematic features (e.g., hand speed, target position) to high frequency ECoG activity (HFA) of each electrode. The two primary findings were: 1) encoding strength (as assessed by held-out R2 values) of ipsilateral and contralateral movements was more bilateral in the left hemisphere than in the right and 2) across-arm generalization was stronger in the left hemisphere than in the right. As the authors point out in the Introduction, there are known 'asymmetries between the two hemispheres in terms of praxis', so it may not be surprising to find asymmetries in the kinematic encoding of the two hemispheres (i.e., the left hemisphere contributes 'more equally' to movements on either side of the body than the right hemisphere).
There is one point that I feel must be addressed before the present conclusions can be reached and a second clarification that I feel will greatly improve the interpretability of the results.
First, as is often the case when working with patients, the authors have no control over the recording sites. This led to some asymmetries in both the number of electrodes in each hemisphere (as the authors note in the Discussion) and (more importantly) in the location of the recording electrodes. Recording site within a hemisphere must be controlled for before any comparisons between the hemispheres can be made. For example, the authors note that 'the contralateral bias becomes weaker the further the electrodes are from putative motor cortex'. If there happen to be more electrodes placed further from M1 in the left hemisphere (as Supplementary Figure 1 seems to suggest), than we cannot know whether the results of Figures 2 and 3 are due to the left hemisphere having stronger bilateral encoding or simply more electrodes placed further from M1.
The reviewer makes a very valid point and this comment has led to our inclusion of a new Supplementary Figure, S2, in which we quantify the percentage of electrodes in each subregion.
Second, it would be useful if the authors provided a bit of clarification about what type of kinematic information the linear model is using to predict HFA. I believe the paragraph titled 'Target modulation and tuning similarity across arms' suggests that there is very little across-target variance in the HFA signal. Does this imply that the model is primarily ignoring the Phi and Theta (as well as their lagged counterparts) and is instead relying on the position and speed terms? How likely is it that the majority of the HFA activity around movement onset reflects a condition-invariant 'trigger signal' (Kaufman, et al., 2016). This trigger signal accounts for the largest portion of neural variance around movement onset (by far), and the weight of individual neurons in trigger signal dimensions tend to be positive, which means that this signal will be strongly reflected in population activity (as measured by ECoG). This interpretation does not detract from the present results in any way, but it may serve to clarify them.
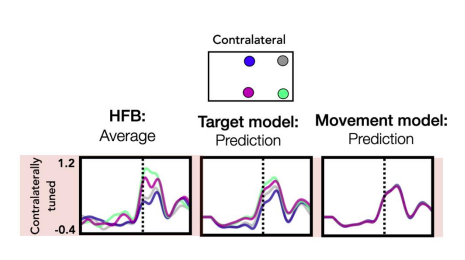
To address this comment, we have added a new figure (Fig 6) which shows the relative contribution of each kinematic feature as well as their average weights across time for both contralateral and ipsilateral movements. This figure also addresses the reviewer’s question about the contribution of the target position to the model. As can be seen, features that reflect timing/movement initiation (position, speed) make a larger contribution compared to the two features which capture directional tuning (theta, phi). As the reviewer suggested, this result is in line Kaufman et al. (2016) which reported that a condition-invariant ‘trigger signal’ comprises the largest component of neural activity. We note that the target dependent features theta and phi still make a substantial contribution to the model (relative contribution: contra = 32%, ipsi = 37%). Previously, we have tested the contribution of the theta and phi features by comparing two models, one that only used position and speed (Movement model) and one that also included the two angular components phi and theta (Target Model). For a subset of electrodes, the held-out predictions were significantly better using the Target Model, a result we take as further evidence of electrode tuning within our dataset.
The figure below shows an electrode located in M1 that is tuned to targets when the patient reached with their contralateral arm as an example. We believe that having an explicit depiction of how the four features contribute to the HFA predictions will help the reader evaluate the model. These points are now addressed in the text in the results section discussing Figure 6.
-

Evaluation Summary:
This paper provides further evidence for hemispheric asymmetry in the cortical control of manual actions based on intracranial (ECoG) recordings in human participants. Specifically, based a linear encoding model, the authors argue that movement encoding is more bilateral in the left hemisphere than the right hemisphere. The paper is well-written and the analyses are largely appropriate for addressing the primary hypothesis, though it would be helpful to detail the variability of electrode placement across individuals (which arises for the clinical intervention being undertaken) and incorporate this variability into the statistical analysis. Given the novelty of this type of human data and the well established question being addressed, this paper will be of interest to both basic and clinical researchers in motor …
Evaluation Summary:
This paper provides further evidence for hemispheric asymmetry in the cortical control of manual actions based on intracranial (ECoG) recordings in human participants. Specifically, based a linear encoding model, the authors argue that movement encoding is more bilateral in the left hemisphere than the right hemisphere. The paper is well-written and the analyses are largely appropriate for addressing the primary hypothesis, though it would be helpful to detail the variability of electrode placement across individuals (which arises for the clinical intervention being undertaken) and incorporate this variability into the statistical analysis. Given the novelty of this type of human data and the well established question being addressed, this paper will be of interest to both basic and clinical researchers in motor neuroscience.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
The findings reported in this manuscript provide support for Liepmann's (1905) seminal observations in apraxia patients in which he proposed lateralization of skilled movement representations in the left hemisphere and identification of the premotor-parietal complex as the principal candidate network involved in the formation of these representations. The prominent role of the left hemisphere in movement control has been documented in several medical imaging studies looking into brain activations associated with dominant and nondominant limb motions as well as bimanual motions. The present study adds new information to this general claim by making use of intracranial recordings (ECoG) in a patient group (n=6, n=665 electrodes meeting inclusion criteria) to explore hemispheric dominance for kinematic encoding …
Reviewer #1 (Public Review):
The findings reported in this manuscript provide support for Liepmann's (1905) seminal observations in apraxia patients in which he proposed lateralization of skilled movement representations in the left hemisphere and identification of the premotor-parietal complex as the principal candidate network involved in the formation of these representations. The prominent role of the left hemisphere in movement control has been documented in several medical imaging studies looking into brain activations associated with dominant and nondominant limb motions as well as bimanual motions. The present study adds new information to this general claim by making use of intracranial recordings (ECoG) in a patient group (n=6, n=665 electrodes meeting inclusion criteria) to explore hemispheric dominance for kinematic encoding in the upper limbs during reaching movements to different targets presented parallel to the frontal plane. Previous single cell recording studies have not provided clear answers to this lateralization question for several reasons, one being the limitations associated with the study of nonhuman primates who exhibit differences in brain lateralization as compared to humans.
The authors looked into movement encoding during an instruction (planning) phase and a movement execution phase and provided compelling evidence for more prominent bilateral encoding in the left than right hemisphere, leading to greater overlap between representations of contralateral and ipsilateral movement in the left hemisphere. The data suggest that the more prominent bilateral encoding in the left hemisphere is already present during the movement preparation phase and further strengthens during the execution phase. This is also associated with better across-arm generalization of neural signals in the left hemisphere. The work also points to a prominent role of left premotor-parietal brain areas for this distinct control architecture. Overall, the study appears to have been conducted with great care in a group of 6 participants and the findings are statistically well supported. The different analyses also provide converging support for bilateral encoding processes in the left hemisphere.
I am sympathetic towards the employed encoding model that makes use of kinematic features to predict neural activity for each electrode in order to retain the high spatial and temporal resolution of the ECoG signal. This approach provides an interesting way to map kinematics to neural activity in a time-resolved manner.
The evidence for more consistent bilateral encoding in the left hemisphere prompts another important question that the authors only address indirectly, i.e., the locus of abstract (effector-independent) representations of movement in the human brain that may also provide the neural architecture for 'motor equivalence', a hallmark of central nervous system flexibility in reaching action goals. To that extent, the current findings may have implications for the neural basis of movement control that may extend beyond the simple reaching movements studied here.
The reported data point to an important role of the premotor and parietal regions of the left as compared to the right hemisphere in the control of ipsilateral and contralateral limb movements. These are also the regions where the electrodes were primarily located in both subgroups of patients. I have 2 concerns in this respect. The first concern refers to the specific locus of these electrodes. For premotor cortex, the authors suggest PMd as well as PMv as potential sites for these bilateral representations. The other principal site refers to parietal cortex but this covers a large territory. It would help if more specific subregions for the parietal cortex can be indicated, if possible. Do the focal regions where electrodes were positioned refer to the superior vs inferior parietal cortex (anterior or posterior), or intra-parietal sulcus. Second, the manuscript's focus on the premotor-parietal complex emerges from the constraints imposed by accessible anatomical locations in the participants but does not preclude the existence of other cortical sites as well as subcortical regions and cerebellum for such bilateral representations. It is meaningful to clarify this and/or list this as a limitation of the current approach.
The evidence for bilateral encoding during unilateral movement opens perspectives for a better understanding of the control of bimanual movements which are abundant during every day life. In the discussion, the authors refer to some imaging studies on bimanual control in order to infer whether the obtained findings may be a consequence of left hemisphere specialization for bimanual movement control, leading to speculations about the information that is being processed for each of both limb movements. Another perspective to consider is the possibility that making a movement with one limb may require postural stabilization in the trunk and contralateral body side, including a contribution from the opposite limb that is supposedly resting on the start button. Have the authors considered whether this postural mechanism could (partly) account for this bilateral encoding mechanism, in particular, because it appears more prominent during movement execution as compared to preparation. Furthermore, could the prominence of bilateral encoding during movement execution be triggered by inflow of sensory information about both limbs from the visual as well as the somatosensory systems.
-

Reviewer #2 (Public Review):
Summary:
Merrick et al. studied kinematic encoding of arm reaching movement in the intracranial recording (ECoG) data in 6 human patients. Of these six patients, 3 had electrodes on the left hemisphere surface, and the rest had electrodes on the right hemisphere surface. They performed an unconstrained instructed delayed-reach task with either the left or the right hand.
The main claim of the paper is that, in right-handers, the left hemisphere, especially premotor and parietal regions, encodes ipsilateral arm movement more strongly than the right hemisphere does. The claim is mostly supported by the results of a set of kinematic encoding model analyses applied to the high-frequency cortical activity (HFA) during the reaching task. First, the prediction accuracies for contra- vs. ipsilateral arm movements …
Reviewer #2 (Public Review):
Summary:
Merrick et al. studied kinematic encoding of arm reaching movement in the intracranial recording (ECoG) data in 6 human patients. Of these six patients, 3 had electrodes on the left hemisphere surface, and the rest had electrodes on the right hemisphere surface. They performed an unconstrained instructed delayed-reach task with either the left or the right hand.
The main claim of the paper is that, in right-handers, the left hemisphere, especially premotor and parietal regions, encodes ipsilateral arm movement more strongly than the right hemisphere does. The claim is mostly supported by the results of a set of kinematic encoding model analyses applied to the high-frequency cortical activity (HFA) during the reaching task. First, the prediction accuracies for contra- vs. ipsilateral arm movements were more equally high for the left hemisphere electrodes than the right hemisphere electrodes. Second, the cross-arm generalization of encoding model weight was also better for the left hemisphere electrodes than the right hemisphere, indicating shared movement representation in the left hemisphere between contra- and ipsilateral limbs. The electrodes with higher cross-arm generalization included the left premotor and parietal areas.
As the authors emphasize, this paper is the first to explicitly compare movement encoding between the two hemispheres in human ECoG recording data and show the left-hemisphere dominance. While the presented results seemingly support the authors' key claims, the details regarding the statistical analyses need to be clarified to judge whether the results generalize to the population.Strengths:
As noted by the authors, the current set of data is valuable for making hemispheric comparisons in human ECoG data; three patients with electrodes implanted on the left hemisphere and the other three patients with electrodes on the right hemisphere.
The choice of encoding modeling approach on each electrode has an advantage over the conventional decoding approach in unambiguously interpreting the contribution of each electrode to the movement as recommended by Kriegeskorte & Douglas (2019). Moreover, the combination of the simple encoding model of each limb's kinetics and the cross-limb generalization test would be a powerful approach to study ipsilateral movement representation, allowing one to dissect "unshared" and "shared" ipsilateral movement representations.
The time-resolved analysis (and the use of sufficient time delays in the kinematic feature matrix in the encoding model) can give further insight into how ipsi- and contralateral movement representations unfold over the course of movement planning and execution.
Weaknesses:
- Although the current human ECoG data set is valuable, there is still large variability in electrode coverage across the patients (I fully acknowledge the difficulty). This makes statistical assessment a bit tricky.
The potential factors of interest in the current study would be Electrode (=Region), Subject, Hemisphere, and their interactions. The tricky part is that Electrode is nested within Subject, and Subject is nested within Hemisphere. Permutation-based ANOVA used for the current paper requires proper treatment of these nested factors when making permutations (Anderson and Braak, 2003). With this regard, sufficient details about how the authors treated each factor, for instance, in each pbANOVA, are not provided in the current version of the manuscript. Similarly, the scope of statistical generalizability, whether the inference is within-sample or population-level, for the claims (e.g., statement about the hemispheric or regional difference) needs to be clarified.
Impact and utility of data:
Whereas this paper is the first to report the left-hemisphere dominance in the movement encoding, the finding itself seems not as striking as seen in the authors' tone of the claim. There have been several previous studies with results implying the asymmetry in the movement representation though not explicitly tested (e.g., Fig. 2B and 5A in Diedrichsen et al., Cereb Cortex, 2013; Fig. 3B in Wiestler and Waters-Metenier et al., J Neurosci, 2014; Figs. 2, 4, and 5 in Haar et al., J Neurosci, 2017), as well as the general notion of the left-hemisphere dominance in praxis, as noted by the authors. That said, the importance of this work in the field is still clear in terms of it being the first evidence of left-hemisphere dominance for arm movement representation in human electrophysiological recording data and the value of the data set available for more detailed analyses in the future.
Additional contexts that would help readers interpret or understand the significance of the work:
The greater amount of shared movement representation in the left hemisphere may imply the greater reliance of the left arm on the left hemisphere. This may, in turn, lead to the greater influence of the ongoing right arm motion on the left arm movement control during the bimanual coordination. Indeed, this point is addressed by the authors in the Discussion (page 15, lines 26-41). One critical piece of literature missing in this context is the work done by Yokoi, Hirashima, and Nozaki (2014). In the experiments using the bimanual reaching task, they in fact found that the learning by the left arm is to the greater degree influenced by the concurrent motion of the right arm than vice versa (Yokoi et al., J Neurosci, 2014). Together with Diedrichsen et al. (2013), this study will strengthen the authors' discussion and help readers interpret the present result of left hemisphere dominance in the context of more skillful bimanual action.
-

Reviewer #3 (Public Review):
In the present work, Merrick et al. analyzed ECoG recordings from patients performing out-and-back reaching movements. The authors trained a linear model to map kinematic features (e.g., hand speed, target position) to high frequency ECoG activity (HFA) of each electrode. The two primary findings were: 1) encoding strength (as assessed by held-out R2 values) of ipsilateral and contralateral movements was more bilateral in the left hemisphere than in the right and 2) across-arm generalization was stronger in the left hemisphere than in the right. As the authors point out in the Introduction, there are known 'asymmetries between the two hemispheres in terms of praxis', so it may not be surprising to find asymmetries in the kinematic encoding of the two hemispheres (i.e., the left hemisphere contributes 'more …
Reviewer #3 (Public Review):
In the present work, Merrick et al. analyzed ECoG recordings from patients performing out-and-back reaching movements. The authors trained a linear model to map kinematic features (e.g., hand speed, target position) to high frequency ECoG activity (HFA) of each electrode. The two primary findings were: 1) encoding strength (as assessed by held-out R2 values) of ipsilateral and contralateral movements was more bilateral in the left hemisphere than in the right and 2) across-arm generalization was stronger in the left hemisphere than in the right. As the authors point out in the Introduction, there are known 'asymmetries between the two hemispheres in terms of praxis', so it may not be surprising to find asymmetries in the kinematic encoding of the two hemispheres (i.e., the left hemisphere contributes 'more equally' to movements on either side of the body than the right hemisphere).
There is one point that I feel must be addressed before the present conclusions can be reached and a second clarification that I feel will greatly improve the interpretability of the results.
First, as is often the case when working with patients, the authors have no control over the recording sites. This led to some asymmetries in both the number of electrodes in each hemisphere (as the authors note in the Discussion) and (more importantly) in the location of the recording electrodes. Recording site within a hemisphere must be controlled for before any comparisons between the hemispheres can be made. For example, the authors note that 'the contralateral bias becomes weaker the further the electrodes are from putative motor cortex'. If there happen to be more electrodes placed further from M1 in the left hemisphere (as Supplementary Figure 1 seems to suggest), than we cannot know whether the results of Figures 2 and 3 are due to the left hemisphere having stronger bilateral encoding or simply more electrodes placed further from M1.
Second, it would be useful if the authors provided a bit of clarification about what type of kinematic information the linear model is using to predict HFA. I believe the paragraph titled 'Target modulation and tuning similarity across arms' suggests that there is very little across-target variance in the HFA signal. Does this imply that the model is primarily ignoring the Phi and Theta (as well as their lagged counterparts) and is instead relying on the position and speed terms? How likely is it that the majority of the HFA activity around movement onset reflects a condition-invariant 'trigger signal' (Kaufman, et al., 2016). This trigger signal accounts for the largest portion of neural variance around movement onset (by far), and the weight of individual neurons in trigger signal dimensions tend to be positive, which means that this signal will be strongly reflected in population activity (as measured by ECoG). This interpretation does not detract from the present results in any way, but it may serve to clarify them.
-