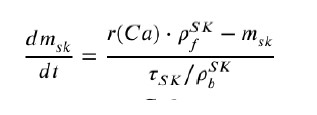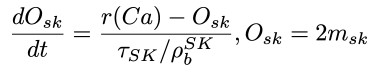A stochastic model of hippocampal synaptic plasticity with geometrical readout of enzyme dynamics
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This paper proposes a computational model that combines biologically detailed elements with more simplified components to provide a comprehensive model of synaptic plasticity. It includes the stochastic character of many of the biophysical processes and introduces a new way to readout the plasticity cascade. It is evaluated against impressively many published experimental studies of hippocampal plasticity. The paper should be of interest not only to computational neuroscience but also to the synaptic neuroscience community but will benefit from a clearer description of assumptions and weaknesses, and a clearer separation of the essential elements in this model from the less critical elements.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Discovering the rules of synaptic plasticity is an important step for understanding brain learning. Existing plasticity models are either (1) top-down and interpretable, but not flexible enough to account for experimental data, or (2) bottom-up and biologically realistic, but too intricate to interpret and hard to fit to data. To avoid the shortcomings of these approaches, we present a new plasticity rule based on a geometrical readout mechanism that flexibly maps synaptic enzyme dynamics to predict plasticity outcomes. We apply this readout to a multi-timescale model of hippocampal synaptic plasticity induction that includes electrical dynamics, calcium, CaMKII and calcineurin, and accurate representation of intrinsic noise sources. Using a single set of model parameters, we demonstrate the robustness of this plasticity rule by reproducing nine published ex vivo experiments covering various spike-timing and frequency-dependent plasticity induction protocols, animal ages, and experimental conditions. Our model also predicts that in vivo-like spike timing irregularity strongly shapes plasticity outcome. This geometrical readout modelling approach can be readily applied to other excitatory or inhibitory synapses to discover their synaptic plasticity rules.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
The work proposes a new computational rule for classifying synaptic plasticity outcome based on the geometry of synaptic enzyme dynamics. Specifically, the authors implement a multi-timescale model of hippocampal synaptic plasticity induction that takes into account the dynamics of the membrane potential, calcium concentration as well as CaMKII and calcineurin signalling pathways. They show that the proposed rule could be applied to reproduce the outcomes from nine published experimental studies involving different spike-timing and frequency-dependent plasticity induction protocols, animal ages, and experimental conditions. The model has been also used to generate predictions regarding the effect of spike-timing irregularity on plasticity outcomes. The proposed approach constitutes an …
Author Response
Reviewer #2 (Public Review):
The work proposes a new computational rule for classifying synaptic plasticity outcome based on the geometry of synaptic enzyme dynamics. Specifically, the authors implement a multi-timescale model of hippocampal synaptic plasticity induction that takes into account the dynamics of the membrane potential, calcium concentration as well as CaMKII and calcineurin signalling pathways. They show that the proposed rule could be applied to reproduce the outcomes from nine published experimental studies involving different spike-timing and frequency-dependent plasticity induction protocols, animal ages, and experimental conditions. The model has been also used to generate predictions regarding the effect of spike-timing irregularity on plasticity outcomes. The proposed approach constitutes an interesting and original idea that contributes to the ongoing effort in discovering the rules of synaptic plasticity.
The conclusions of this paper are mostly well supported by data, but some model assumptions and interpretation of modelling results need to be clarified and extended.
- The proposed model captures well the stochastic nature of the dendritic spine ion channels and receptors except for the calcium-sensitive potassium (SK) channel that has been modelled deterministically. Given that the same justification in terms of small number of channels present in the small dendritic spine compartment applies to the SK channels as well as to the voltage gated calcium channels and the AMPA and NMDA receptors, it is not clear why the authors have chosen a deterministic representation in the case of SK. The implications of this assumption needs to be investigated and discussed.
There are several stochastic models of AMPA and NMDA receptors based on single-channel recordings. Additionally, we had enough experimental data on single channel recordings to build a custom Markov chain model of VGCCs. For the SK channel, we could not find enough experimental data (age-dependence activity, temperature sensitivity, etc.) to custom-build a stochastic model. We thus decided to implement a deterministic model. Yet, we understand the reviewers’ comment that in theory, a stochastic model of SK channels could impact our results. We thus now provide a simulation with a stochastic model of SK, comparing it to the deterministic model implemented in the study.
We describe a minimal version of a stochastic model of SK compatible with the deterministic version. The deterministic model of SK channel fit at ~35C is described in the methods section.
Because of the factor ρ 𝑓𝑆𝐾 in the equation, which multiplies r(Ca) by ~2, the equation cannot be related to a 2-state Markov chain (MC). This could probably be possible with a 3-state MC but we used a different strategy. Noting that ρ 𝑆𝐾 ∼ 2 , we introduce a new equation
As 0 < r(Ca) < 1, it is straightforward to introduce a 2-state MC for which the above equation describes the probability of the open state. We then simulate two such independent (for a given Ca concentration) channels and approximate 𝑚 𝑆𝐾 as the sum (which belongs to [0,2Nsk]) of the open states for the 2 channels.
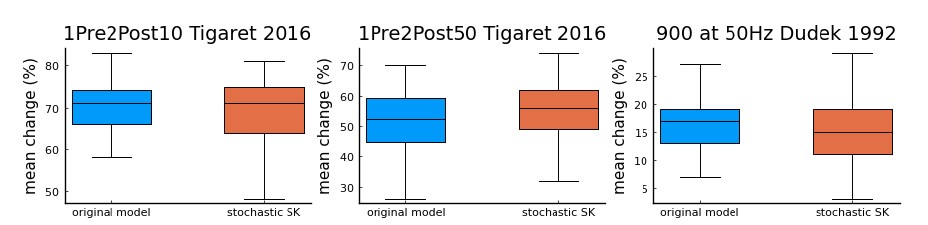
As the reviewer can see in the figure below, we do not find a major difference in the simulations of 3 protocols. Thus, we argue that adding a stochastic version of the SK channels in our current study would not fundamentally alter our main conclusions.
Figure Legend: a comparison using Tigaret et al. 2016 1Pre2Post10 and 1Pre2Post50 protocols, and 900 at 50 Hz protocol from Dudek and Bear 1992 (100 repetitions) between the model with the deterministic SK channel (original model - blue), and the modified model including the stochastic SK channel (stochastic SK - red). Deterministic vs stochastic SK channel does not significantly modify the model’s behaviour.
To explain our rationale of using a deterministic version of SK channel, we provide this sentence in the Methods when describing SK channel model: “"Due to a lack of single-channel recordings of SK channels, and a lack of published stochastic models of SK channels, we modelled SK channels deterministically. In tests we found that this assumption had only a negligible impact on the outcomes of plasticity protocols (data not shown)" (page 40).
- Many of the model parameters have been set to values previously estimated from synaptic physiology and biochemistry experiments, However, a significant number of important parameter values have been tuned to reproduce the plasticity experiments targeted in this study. As such, it needs to be explained which of the plasticity outcomes have been reproduced because the parameters are chosen to do so. A clarification would have helped to substantiate the authors' conclusions.
Most parameters were set with values previously defined by experimental work. We referred to these publications where necessary throughout the Methods and Tables in our original manuscript. For the few free parameters that were adjusted, we now provide additional information wherever necessary for the Tables concerned.
● In the legend of Table 4 (neuron electrical properties), we explain which parameters are different from values obtained from the literature to fit experimental data (Golding et al. 2001; Buchanan et al. 2007).
● Parameters for the sodium and potassium conductance (Table 5) are labelled as generic since they are intentionally set to produce the BaP dynamics we have shown in the paper.
● Table 6 has no free parameters.
● Table 7 caption now includes a description saying ’Note that the buffer concentration, calcium diffusion coefficient, calcium diffusion time constant and calcium permeability were considered free parameters to adjust the calcium dynamics’.
● In Table 8 we had originally pointed out how we adapted the GluN2B rates from a published GluN2A model (Popescu et al. 2004; and Iacobucci and Popesco 2018). We now describe this adaptation in the Table 8 legend. In this Table, we now also better explain how we adjusted the NMDAr model to reflect the ratio between GluN2B and GluN2A, fitted from Sinclair et al. 2016; and the NMDAr conductance depending on calcium fitted from Maki and Popescu 2014.
● In Table 9 caption we now explain how the GABAr number and conductance were modified to fit GABAr currents as in Figures 15 b and e. The relevant parameters are indicated in the table.
● In Table 10 caption we now state the number of VGCCs per subtype that we used as a free parameter to reproduce the calcium dynamics (Figure 12).
- Adding experimental testing of model predictions, for example, that firing variability can alter the rules of plasticity, in the sense that it is possible to add noise to cause LTP for protocols that did not otherwise induce plasticity would be needed to increase confidence in the presented modelling results.
We agree that it would be interesting in the future to test the many model predictions suggested in this work with biological experiments. This would however require a lot of work and will be the subject of further studies.
Reviewer #3 (Public Review):
This manuscript presents and analyzes a novel calcium-dependent model of synaptic plasticity combining both presynaptic and postsynaptic mechanisms, with the goal of reproducing a very broad set of available experimental studies of the induction of long-term potentiation (LTP) vs. long-term depression (LTD) in a single excitatory mammalian synapse in the hippocampus. The stated objective is to develop a model that is more comprehensive than the often-used simplified phenomenological models, but at the same time to avoid biochemical modeling of the complex molecular pathways involved in LTP and LTD, retaining only its most critical elements. The key part of this approach is the proposed "geometric readout" principle, which allows to predict the induction of LTP vs. LTD by examining the concentration time course of the two enzymes known to be critical for this process, namely (1) the Ca2+/calmodulin-bound calcineurin phosphatase (CaN), and (2) the Ca2+/calmodulin-bound protein kinase (CaMKII). This "geometric readout" approach bypasses the modeling of downstream pathways, implicitly assuming that no further biochemical information is required to determine whether LTP or LTD (or no synaptic change) will arise from a given stimulation protocol. Therefore, it is assumed that the modeling of downstream biochemical targets of CaN and CaMKII can be avoided without sacrificing the predictive power of the model. Finally, the authors propose a simplified phenomenological Markov chain model to show that such "geometric readout" can be implemented mechanistically and dynamically, at least in principle.
Importantly, the presented model has fully stochastic elements, including stochastic gating of all channels, stochastic neurotransmitter release and stochastic implementation of all biochemical reactions, which allows to address the important question of the effect of intrinsic and external noise on the induction of LTP and LTD, which is studied in detail in this manuscript.
Mathematically, this modeling approach resembles a continuous stochastic version of the "liquid computing" / "reservoir computing" approach: in this case the "hidden layer", or the reservoir, consists of the CaMKII and CaM concentration variables. In this approach, the parameters determining the dynamics of these intermediate ("hidden") variables are kept fixed (here, they are constrained by known biophysical studies), while the "readout" parameters are being trained to predict a target set of experimental observations.
Strengths:
- This modeling effort is very ambitious in trying to match an extremely broad array of experimental studies of LTP/LTD induction, including the effect of several different pre- and post-synaptic spike sequence protocols, the effect of stimulation frequency, the sensitivity to extracellular Ca2+ and Mg2+ concentrations and temperature, the dependence of LTP/LTD induction on developmental state and age, and its noise dependence. The model is shown to match this large set of data quite well, in most cases.
- The choice for stochastic implementation of all parts of the model allows to fully explore the effects of intrinsic and extrinsic noise on the induction of LTP/LTD. This is very important and commendable, since regular noise-less spike firing induction protocols are not very realistic, and not every relevant physiologically.
- The modeling of the main players in the biochemical pathways involved in LTP/LTD, namely CaMKII and CaN, aims at sufficient biological realism, and as noted above, is fully stochastic, while other elements in the process are modeled phenomenologically to simplify the model and reveal more clearly the main mechanism underlying the LTP/LTD decision switch.
- There are several experimentally verifiable predictions that are proposed based on an in-depth analysis of the model behavior.
We thank the reviewer for pointing out these strengths.
Weaknesses:
- The stated explicit goal of this work is the construction of a model with an intermediate level of detail, as compared to simplified "one-dimensional" calcium-based phenomenological models on the one hand, and comprehensive biochemical pathway models on the other hand. However, the presented model comes across as extremely detailed nonetheless. Moreover, some of these details appear to be avoidable and not critical to this work. For instance, the treatment of presynaptic neurotransmitter release is both overly detailed and not sufficiently realistic: namely, the extracellular Ca2+ concentration directly affects vesicle release probability but has no effect on the presynaptic calcium concentration. I believe that the number of parameters and the complexity in the presynaptic model could be reduced without affecting the key features and findings of this work.
This point is largely answered in Essential Revisions point 4 where we argue the choices we made for the presynaptic model. We acknowledge, however, that in this current version, we did not incorporate all biophysical components, such as the modulation of presynaptic calcium concentration with external calcium variations and multivesicular release. The calcium-dependence of presynaptic release, as modeled currently, is however fitted in Figure 8e against data from Hardingham et al. 2006 and Tigaret et al. 2016. These current limitations could be addressed in a next version of our presynaptic model where we also plan to incorporate age and temperature influence.
- The main hypotheses and assumptions underlying this work need to be stated more explicitly, to clarify the main conclusions and goals of this modeling work. For instance, following much prior work, the presented model assumes that a compartment-based (not spatially-resolved) model of calcium-triggered processes is sufficient to reproduce all known properties of LTP and LTD induction and that neither spatially-resolved elements nor calcium-independent processes are required to predict the observed synaptic change. This could be stated more explicitly. It could also be clarified that the principal assumption underlying the proposed "geometric readout" mechanisms is that all information determining the induction of LTP vs. LTP is contained in the time-dependent spine-averaged Ca2+/calmodulin-bound CaN and CaMKII concentrations, and that no extra elements are required. Further, since both CaN and CaMKII concentrations are uniquely determined by the time course of postsynaptic Ca2+ concentration, the model implicitly assumes that the LTP/LTD induction depends solely on spine-averaged Ca2+ concentration time course, as in many prior simplified models. This should be stated explicitly to clarify the nature of the presented model.
We thank the reviewer for the suggestions on how to clarify the main hypotheses and assumptions of our work. We slightly modified the sentences provided by the reviewer and added them in the main text (page 2, lines 82 and page 19, lines 593).
- In the Discussion, the authors appear to be very careful in framing their work as a conceptual new approach in modeling STD/STP, rather than a final definitive model: for instance, they explicitly discuss the possibility of extending the "geometric readout" approach to more than two time-dependent variables, and comment on the potential non-uniqueness of key model parameters. However, this makes it hard to judge whether the presented concrete predictions on LTP/LTD induction are simply intended as illustrations of the presented approach, or whether the authors strongly expect these predictions to hold. The level of confidence in the concrete model predictions should be clarified in the Discussion. If this confidence level is low, that would call into question the very goal of such a modeling approach.
These are very good questions. Let us first comment on the parameter uniqueness. We believe, like in E. Marder’s work on ion channels expression in neurons, that the synapse has the possibility to adapt its internal parameters (proteins number, transition rates, etc) to provide a given functioning behaviour. As a by-product, there is non uniqueness of parameters associated with behavior. Additionally, since our model is able to reproduce 9 published experimental outcomes with a single set of parameters, it is a functioning synapse with adjusted parameters which output the expected behaviours. Thus by extrapolation, our confidence in the further predictions is high. We modified sentences in the discussion section to argue this point (page 21, line 707).
Let us comment now on increasing the complexity. To our best, we strived to design a plasticity readout as simple as possible yet providing a functioning synapse. Given our success to reproduce 9 published experimental outcomes with a single set of parameters, adding more complexity would be akin to overfitting.
- The authors presented a simplified mechanistic dynamical Markov chain process to prove that the "geometric readout" step is implementable as a dynamical process, at least in principle. However, a more realistic biochemical implementation of the proposed "region indicator" variables may be complex and not guaranteed to be robust to noise. While the authors acknowledge and touch upon some of these issues in their discussion, it is important that the authors will prove in future work that the "geometric readout" is implementable as a biochemical reaction network. Barring such implementation, one must be extra careful when claiming advantages of this approach as compared to modeling work that attempts to reconstruct the entire biochemical pathways of LTP/LTD induction.
We acknowledge this issue and agree this would be an interesting subject for future work.
-

-

Evaluation Summary:
This paper proposes a computational model that combines biologically detailed elements with more simplified components to provide a comprehensive model of synaptic plasticity. It includes the stochastic character of many of the biophysical processes and introduces a new way to readout the plasticity cascade. It is evaluated against impressively many published experimental studies of hippocampal plasticity. The paper should be of interest not only to computational neuroscience but also to the synaptic neuroscience community but will benefit from a clearer description of assumptions and weaknesses, and a clearer separation of the essential elements in this model from the less critical elements.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive …
Evaluation Summary:
This paper proposes a computational model that combines biologically detailed elements with more simplified components to provide a comprehensive model of synaptic plasticity. It includes the stochastic character of many of the biophysical processes and introduces a new way to readout the plasticity cascade. It is evaluated against impressively many published experimental studies of hippocampal plasticity. The paper should be of interest not only to computational neuroscience but also to the synaptic neuroscience community but will benefit from a clearer description of assumptions and weaknesses, and a clearer separation of the essential elements in this model from the less critical elements.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

-

Reviewer #1 (Public Review):
This paper introduces a detailed computational model for synaptic plasticity, that is innovative in a number of ways. First, it includes the stochastic character of many of the biophysical processes. Second, it introduces a new way to readout the plasticity cascade. Third, it fits a number of experiments that previous models could not fit. It is a complicated model and presents a step forward towards a realistic model of synaptic plasticity. The readout mechanism is artificial but does the job well.
-

-

Reviewer #2 (Public Review):
The work proposes a new computational rule for classifying synaptic plasticity outcome based on the geometry of synaptic enzyme dynamics. Specifically, the authors implement a multi-timescale model of hippocampal synaptic plasticity induction that takes into account the dynamics of the membrane potential, calcium concentration as well as CaMKII and calcineurin signalling pathways. They show that the proposed rule could be applied to reproduce the outcomes from nine published experimental studies involving different spike-timing and frequency-dependent plasticity induction protocols, animal ages, and experimental conditions. The model has been also used to generate predictions regarding the effect of spike-timing irregularity on plasticity outcomes. The proposed approach constitutes an interesting and …
Reviewer #2 (Public Review):
The work proposes a new computational rule for classifying synaptic plasticity outcome based on the geometry of synaptic enzyme dynamics. Specifically, the authors implement a multi-timescale model of hippocampal synaptic plasticity induction that takes into account the dynamics of the membrane potential, calcium concentration as well as CaMKII and calcineurin signalling pathways. They show that the proposed rule could be applied to reproduce the outcomes from nine published experimental studies involving different spike-timing and frequency-dependent plasticity induction protocols, animal ages, and experimental conditions. The model has been also used to generate predictions regarding the effect of spike-timing irregularity on plasticity outcomes. The proposed approach constitutes an interesting and original idea that contributes to the ongoing effort in discovering the rules of synaptic plasticity.
The conclusions of this paper are mostly well supported by data, but some model assumptions and interpretation of modelling results need to be clarified and extended.
The proposed model captures well the stochastic nature of the dendritic spine ion channels and receptors except for the calcium-sensitive potassium (SK) channel that has been modelled deterministically. Given that the same justification in terms of small number of channels present in the small dendritic spine compartment applies to the SK channels as well as to the voltage gated calcium channels and the AMPA and NMDA receptors, it is not clear why the authors have chosen a deterministic representation in the case of SK. The implications of this assumptions needs to be investigated and discussed.
Many of the model parameters have been set to values previously estimated from synaptic physiology and biochemistry experiments, However, a significant number of important parameter values have been tuned to reproduce the plasticity experiments targeted in this study. As such, it needs to be explained which of the plasticity outcomes have been reproduced because the parameters are chosen to do so. A clarification would have helped to substantiate the authors' conclusions.
Adding experimental testing of model predictions, for example, that firing variability can alter the rules of plasticity, in the sense that it is possible to add noise to cause LTP for protocols that did not otherwise induce plasticity would be needed to increase confidence in the presented modelling results.
-

-

Reviewer #3 (Public Review):
This manuscript presents and analyzes a novel calcium-dependent model of synaptic plasticity combining both presynaptic and postsynaptic mechanisms, with the goal of reproducing a very broad set of available experimental studies of the induction of long-term potentiation (LTP) vs. long-term depression (LTD) in a single excitatory mammalian synapse in the hippocampus. The stated objective is to develop a model that is more comprehensive than the often-used simplified phenomenological models, but at the same time to avoid biochemical modeling of the complex molecular pathways involved in LTP and LTD, retaining only its most critical elements. The key part of this approach is the proposed "geometric readout" principle, which allows to predict the induction of LTP vs. LTD by examining the concentration time …
Reviewer #3 (Public Review):
This manuscript presents and analyzes a novel calcium-dependent model of synaptic plasticity combining both presynaptic and postsynaptic mechanisms, with the goal of reproducing a very broad set of available experimental studies of the induction of long-term potentiation (LTP) vs. long-term depression (LTD) in a single excitatory mammalian synapse in the hippocampus. The stated objective is to develop a model that is more comprehensive than the often-used simplified phenomenological models, but at the same time to avoid biochemical modeling of the complex molecular pathways involved in LTP and LTD, retaining only its most critical elements. The key part of this approach is the proposed "geometric readout" principle, which allows to predict the induction of LTP vs. LTD by examining the concentration time course of the two enzymes known to be critical for this process, namely (1) the Ca2+/calmodulin-bound calcineurin phosphatase (CaN), and (2) the Ca2+/calmodulin-bound protein kinase (CaMKII). This "geometric readout" approach bypasses the modeling of downstream pathways, implicitly assuming that no further biochemical information is required to determine whether LTP or LTD (or no synaptic change) will arise from a given stimulation protocol. Therefore, it is assumed that the modeling of downstream biochemical targets of CaN and CaMKII can be avoided without sacrificing the predictive power of the model. Finally, the authors propose a simplified phenomenological Markov chain model to show that such "geometric readout" can be implemented mechanistically and dynamically, at least in principle.
Importantly, the presented model has fully stochastic elements, including stochastic gating of all channels, stochastic neurotransmitter release and stochastic implementation of all biochemical reactions, which allows to address the important question of the effect of intrinsic and external noise on the induction of LTP and LTD, which is studied in detail in this manuscript.
Mathematically, this modeling approach resembles a continuous stochastic version of the "liquid computing" / "reservoir computing" approach: in this case the "hidden layer", or the reservoir, consists of the CaMKII and CaM concentration variables. In this approach, the parameters determining the dynamics of these intermediate ("hidden") variables are kept fixed (here, they are constrained by known biophysical studies), while the "readout" parameters are being trained to predict a target set of experimental observations.
Strengths:
This modeling effort is very ambitious in trying to match an extremely broad array of experimental studies of LTP/LTD induction, including the effect of several different pre- and post-synaptic spike sequence protocols, the effect of stimulation frequency, the sensitivity to extracellular Ca2+ and Mg2+ concentrations and temperature, the dependence of LTP/LTD induction on developmental state and age, and its noise dependence. The model is shown to match this large set of data quite well, in most cases.
The choice for stochastic implementation of all parts of the model allows to fully explore the effects of intrinsic and extrinsic noise on the induction of LTP/LTD. This is very important and commendable, since regular noise-less spike firing induction protocols are not very realistic, and not every relevant physiologically.
The modeling of the main players in the biochemical pathways involved in LTP/LTD, namely CaMKII and CaN, aims at sufficient biological realism, and as noted above, is fully stochastic, while other elements in the process are modeled phenomenologically to simplify the model and reveal more clearly the main mechanism underlying the LTP/LTD decision switch.
There are several experimentally verifiable predictions that are proposed based on an in-depth analysis of the model behavior.
Weaknesses:
The stated explicit goal of this work is the construction of a model with an intermediate level of detail, as compared to simplified "one-dimensional" calcium-based phenomenological models on the one hand, and comprehensive biochemical pathway models on the other hand. However, the presented model comes across as extremely detailed nonetheless. Moreover, some of these details appear to be avoidable and not critical to this work. For instance, the treatment of presynaptic neurotransmitter release is both overly detailed and not sufficiently realistic: namely, the extracellular Ca2+ concentration directly affects vesicle release probability but has no effect on the presynaptic calcium concentration. I believe that the number of parameters and the complexity in the presynaptic model could be reduced without affecting the key features and findings of this work.
The main hypotheses and assumptions underlying this work need to be stated more explicitly, to clarify the main conclusions and goals of this modeling work. For instance, following much prior work, the presented model assumes that a compartment-based (not spatially-resolved) model of calcium-triggered processes is sufficient to reproduce all known properties of LTP and LTD induction and that neither spatially-resolved elements nor calcium-independent processes are required to predict the observed synaptic change. This could be stated more explicitly. It could also be clarified that the principal assumption underlying the proposed "geometric readout" mechanisms is that all information determining the induction of LTP vs. LTP is contained in the time-dependent spine-averaged Ca2+/calmodulin-bound CaN and CaMKII concentrations, and that no extra elements are required. Further, since both CaN and CaMKII concentrations are uniquely determined by the time course of postsynaptic Ca2+ concentration, the model implicitly assumes that the LTP/LTD induction depends solely on spine-averaged Ca2+ concentration time course, as in many prior simplified models. This should be stated explicitly to clarify the nature of the presented model.
In the Discussion, the authors appear to be very careful in framing their work as a conceptual new approach in modeling STD/STP, rather than a final definitive model: for instance, they explicitly discuss the possibility of extending the "geometric readout" approach to more than two time-dependent variables, and comment on the potential non-uniqueness of key model parameters. However, this makes it hard to judge whether the presented concrete predictions on LTP/LTD induction are simply intended as illustrations of the presented approach, or whether the authors strongly expect these predictions to hold. The level of confidence in the concrete model predictions should be clarified in the Discussion. If this confidence level is low, that would call into question the very goal of such a modeling approach.
The authors presented a simplified mechanistic dynamical Markov chain process to prove that the "geometric readout" step is implementable as a dynamical process, at least in principle. However, a more realistic biochemical implementation of the proposed "region indicator" variables may be complex and not guaranteed to be robust to noise. While the authors acknowledge and touch upon some of these issues in their discussion, it is important that the authors will prove in future work that the "geometric readout" is implementable as a biochemical reaction network. Barring such implementation, one must be extra careful when claiming advantages of this approach as compared to modeling work that attempts to reconstruct the entire biochemical pathways of LTP/LTD induction.
-