Optogenetic inhibition of actomyosin reveals mechanical bistability of the mesoderm epithelium during Drosophila mesoderm invagination
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This manuscript studies a topic of interest to developmental biologists using a combination of optogenetics, biophysical concepts, and mathematical modeling. How in plane contractile forces cause out of plane shape changes is a relevant open question, and the optogenetic tools developed in this work provide a new strategy to address the question.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Apical constriction driven by actin and non-muscle myosin II (actomyosin) provides a well-conserved mechanism to mediate epithelial folding. It remains unclear how contractile forces near the apical surface of a cell sheet drive out-of-the-plane bending of the sheet and whether myosin contractility is required throughout folding. By optogenetic-mediated acute inhibition of actomyosin, we find that during Drosophila mesoderm invagination, actomyosin contractility is critical to prevent tissue relaxation during the early, ‘priming’ stage of folding but is dispensable for the actual folding step after the tissue passes through a stereotyped transitional configuration. This binary response suggests that Drosophila mesoderm is mechanically bistable during gastrulation. Computer modeling analysis demonstrates that the binary tissue response to actomyosin inhibition can be recapitulated in the simulated epithelium that undergoes buckling-like deformation jointly mediated by apical constriction in the mesoderm and in-plane compression generated by apicobasal shrinkage of the surrounding ectoderm. Interestingly, comparison between wild-type and snail mutants that fail to specify the mesoderm demonstrates that the lateral ectoderm undergoes apicobasal shrinkage during gastrulation independently of mesoderm invagination. We propose that Drosophila mesoderm invagination is achieved through an interplay between local apical constriction and mechanical bistability of the epithelium that facilitates epithelial buckling.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
This work raises the question of how in plane forces generated at the apical surface of an epithelial cell sheet cause out of plane motion, an important morphogenetic motif. To address this question, a new ontogenetic dominant negative rho1 tool, based on the cry2-CIBN system is presented. The authors use this tool to analyze the well studied biophysical process of ventral furrow formation, and dissect the spatiotemporal requirement of rho1 signaling to modulate myosin accumulation. They separate the effect on morphogenesis into an early phase that becomes significantly slowed down by myosin inhibition, and a late phase where the kinetics is comparable to wild type despite treatment. For interpretation of the data, an older model of cell mechanics treating tissue as a purely elastic …
Author Response
Reviewer #1 (Public Review):
This work raises the question of how in plane forces generated at the apical surface of an epithelial cell sheet cause out of plane motion, an important morphogenetic motif. To address this question, a new ontogenetic dominant negative rho1 tool, based on the cry2-CIBN system is presented. The authors use this tool to analyze the well studied biophysical process of ventral furrow formation, and dissect the spatiotemporal requirement of rho1 signaling to modulate myosin accumulation. They separate the effect on morphogenesis into an early phase that becomes significantly slowed down by myosin inhibition, and a late phase where the kinetics is comparable to wild type despite treatment. For interpretation of the data, an older model of cell mechanics treating tissue as a purely elastic material is presented. It fails to reproduce the observations. As a modification, in analogy to buckling of a thin beam under load, a compressive stress exerted by the adjacent ectoderm is introduced. Further analysis of cell behaviors in response to various laser mediated tissue manipulations is presented as support of the proposed mechanism.
Overall, the manuscript addresses an important aspect of morphogenesis. In particular the use of optogenetic tools promises new insights that might be more challenging to achieve with traditional mutant analysis. However, reservations remain with respect to (1) rigor of the analysis, and (2) interpretation and quality of the data in support of the proposed mechanism; this applies in particular to presentation of biophysical observations, including experiment and simulations.
The manuscript adds valuable quantitative data, in particular the findings described in Fig 2ab. However, insufficient analysis are performed to fully support the claims of the manuscript by the data presented.
(I) The manuscript proposes an elasticity based model of tissue mechanics, but provides no experimental evidence in support of this assumption. Many rheology studies performed in a wide range of specimen (including the Drosophila embryo) found a separation of time scales, that shows elasticity is a good approximation of tissue mechanics only for time scales short compared to the process studied here.
We agree with the reviewer that an elasticity-based model of tissue mechanics is a simplification for the actual tissue properties in the real embryos. To provide justification for this simplification, in the revised manuscript, we have cited a previous biophysical study measuring tissue viscoelasticity in early Drosophila embryos (Doubrovinski et al., 2017). Using a magnetic tweezers-based approach, Doubrovinski et al. shows that the lower bound of the decay time of the elastic response is four minutes (the lower limit on the timescales where tissue behaves elastically). In addition, when history dependence of the response is considered, the decay time increases to nine minutes, which is close to the duration of ventral furrow formation (~ 15 – 20 minutes). Therefore, we consider elasticity is a reasonable approximation of tissue mechanics during ventral furrow formation. The elasticity assumption has been widely used in the previously published modeling work to simulate ventral furrow formation (Allena et al., 2010; Conte et al., 2009; Gracia et al., 2019; Heer et al., 2017; Hocevar Brezavšček et al., 2012; Muñoz et al., 2007; Rauzi et al., 2015).The modeling framework used in our current study, which is initially described in Polyakov et al. 2014, successfully predicts the intermediate and final furrow morphologies with a minimal set of active and passive forces without prescribing individual cell shape changes. It is therefore advantageous to use this model to explore the main novel aspect of the folding mechanics underlying ventral furrow formation. We show that the model can recapitulate the binary tissue response to acute myosin inhibition. In addition, it accurately predicts the intermediate furrow morphology at the transitional state and several other morphological properties associated with myosin inhibition. We therefore believe that this minimalistic model captures the central aspect of the physical mechanism underlying mesoderm bistability observed in the experiments.
(II) The manuscript uses a method of micro-dissection to soften cells, but does not provide a clear definition of the concept softening, provides no rational for the methods functioning, and does not provide independent validation. The described treatment might affect cells in many alternative ways to the offered interpretation. This data is the central experimental evidence given in support of the proposed ectoderm compression mechanism, and therefore it is essential to provide a precise physical explanation of the method, and validation of measurements that bolster the conclusion.
We apologize for not explaining the meaning of “softening” clearly in our original manuscript and the rationale for using laser ablation to detect compression. By “softening”, we meant to describe the mechanical status of the cell when the subcellular structures that normally support the mechanical integrity (e.g., cortical actin) are disrupted. We reason that when such a change in mechanical properties happens in a specific region of a tissue that is under compression, the cells in this region should have an impaired ability to resist compression from outside of the region and thereby cause the region to shrink.
Laser ablation has been widely used to measure tensile stresses in cells and tissues by disruption of cells or subcellular structures. The method we used is adapted from previous described protocols, where a femtosecond near infrared laser is used to disrupt subcellular structures for detection of tissue tension (Rauzi et al., 2015; Rauzi et al., 2008).It has been shown that when laser intensity is properly controlled, the treatment can leave the plasma membrane intact but disrupt subcellular structures associated with the plasma membrane, such as adherens junctions and the cortical actomyosin networks (Rauzi et al., 2015; Rauzi et al., 2008).Using a femtosecond near infrared laser, we were able to ablate embryonic tissues that are under tension and observe tissue recoil after laser ablation, suggesting that our approach has disrupted the cortical cytoskeleton in the laser treated region (e.g., Figure 3 and Authors’ Response Figure 1). In these experiments, the lack of damage on the plasma membrane is indicated by the readily recovery of the plasma membrane signal after laser treatment, as well as the lack of bright burn marks on the tissue.
As we noted before, we reasoned that if tissue is compressive, similar laser treatment that generates tissue recoil in tissues under tension should result in tissue shrinking within the laser-treated region. The data presented in our original manuscript demonstrate that tissue shrinking is not a non-specific response to our laser treatment – we did not observe such a response when we treat the tissue during cellularization or within the first five minutes of gastrulation, although identical experimental conditions were used (Original Figure 4). We have also obtained additional evidence that supports the use of tissue shrinking as a readout of tissue compression. We tested our laser ablation approach in Stage 8 – 9 embryos at regions where cells are actively dividing/proliferating, which would expect to generate compressive stresses in the tissue. As we perform laser ablation in this region, we observed shrinking of the treated region, which was distinct from the tensile tissue response (Authors’ Response Figure 1). While this preliminary evidence is encouraging, we agree with the reviewer that further independent validations are needed given that the methods for detecting tissue compression have not been well established in the field. Following the editor’s suggestion, we have removed this experiment from the current manuscript and focus on the characterization of the optogenetic tool and the binary tissue response after acute actomyosin inhibition.
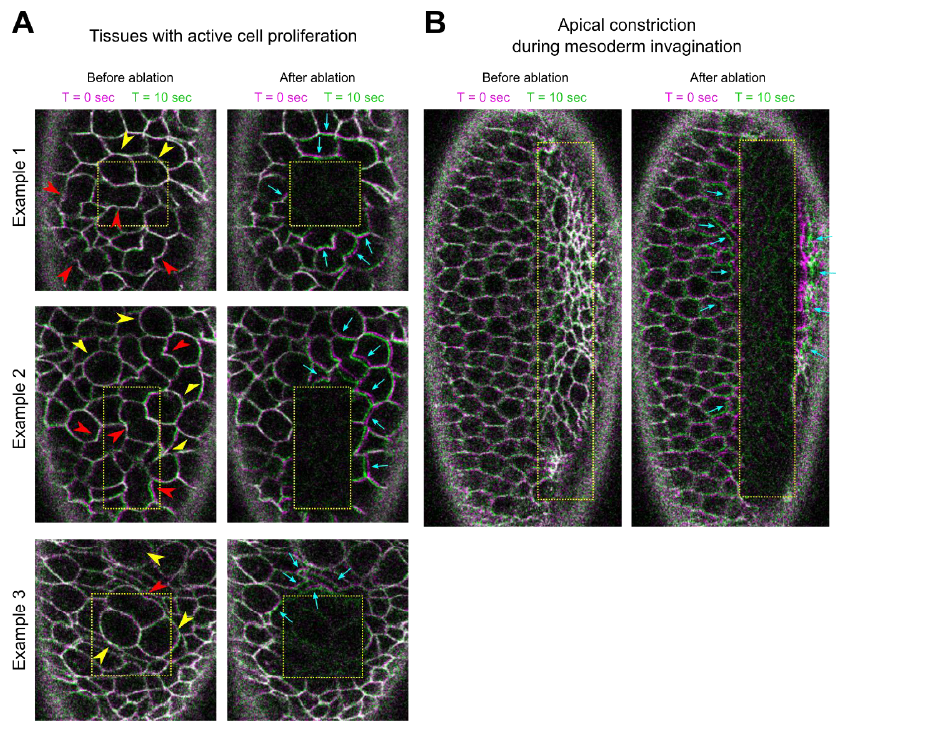
Authors’ Response Figure 1: Laser ablation in regions of tissues with active cell proliferation (a) or undergoing apical constriction (b). The movement of tissues is indicated by overlaying membrane signals (Ecadherin-GFP) at T = 0 sec and at T = 10 sec. T = 0 in the “After ablation” panels marks the time immediately after ablation. (a) Stage 8 – 9 embryos. Multiple cells are in the process of cell division, as indicated by mitotic rounding (yellow arrowheads) or the appearance of cleavage furrows (red arrowheads). Immediately after laser ablation, the surrounding cells moved towards the ablated region (cyan arrows). (b) An embryo undergoing ventral furrow formation. Ablation within the constriction domain results in recoil of the surrounding cells away from the ablated region (cyan arrows).
(III) Mechanical isolation of the mesoderm is a very exciting approach to test the possible involvement of adjacent tissues in folding. Indeed, the authors report a delay of ventral furrow formation. However, there is no evidence provided that (a) the mesoderm is mechanically uncoupled, and (b) that the treatment did not have undesired side effects. For example, a similar procedure (so-called cauterization, see Rauzi 2015) has been used to immobilize cells in the Drosophila embryo. Such an effect could account for the observed delay in furrow formation.
We agree with the reviewer that “mechanical uncoupling” is merely a prediction based on our observation but has not been directly demonstrated. On the other hand, since the purpose of this experiment is to ask whether the presence of the lateral ectoderm is important for the mesoderm to transition between apical constriction and invagination (and our result shows yes), whether the approach we used mechanically uncoupled mesoderm and the ectoderm is no longer an immediately relevant question. We apologize for the imprecise use of the term “mechanically uncoupling” in our original manuscript and we thank the reviewer for pointing this out.
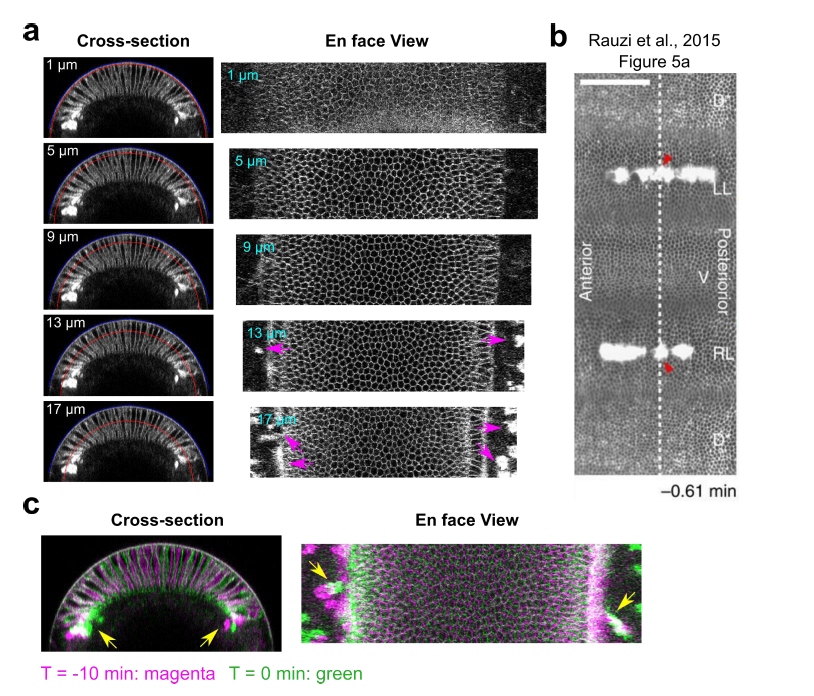
As for the reviewer’s point (b), we have several pieces of evidence indicating that our approach did not cause anchoring of the tissue to the vitelline membrane. The major difference between the approach we used and that used by Rauzi et al. 2015 is the location of the tissue where the laser treatment was imposed. In order to anchor the tissue to the vitelline membrane, Rauzi et al. target the laser to the apical side of the tissue, adjacent to the vitelline membrane. The resulting cauterization of the tissue caused anchoring of the tissue to the vitelline membrane, presumably by fusion of the tissue with the vitelline membrane. In our approach, we used similar type of laser (femtosecond near infrared laser) to perform tissue disruption, but instead of targeting the apical side of the tissue, we targeted the basal region of the invaginating cleavage furrows during cellularization, with the goal to block cell formation. While the laser intensity we used is high enough to cause cauterization of the tissue as indicated by the appearance of bright autofluorescence in the laser treated region, these “burn marks” are not located at the apical side of the cells (Authors’ Response Figure 2a). The lack of “burn marks” on the vitelline membrane in our experiment is in sharp contrast to the result shown in Rauzi et al 2015 (see Authors’ Response Figure 2b for an example from Rauzi et al in comparison to our own data in 2a). Because of the difference in the location of cauterization, we do not expect that the tissue would be fused with the vitelline membrane after our treatment. This is further suggested by the observation that the burn marks can move before the onset of gastrulation, which again indicates that the tissue is not anchored to the vitelline membrane (Authors’ Response Figure 2c).
That being said, we acknowledge that we do not fully understand the impact of the laser treatment on the embryo (e.g., what causes the reduced rate of apical constriction), and more control experiments are required in order to fully describe the tissue response we observed. As suggested by the editor, we decided to remove the ectoderm-ablation experiment from the revised manuscript and focus on the characterization of the optogenetic tool and the binary tissue response after acute actomyosin inhibition.
Authors’ Response Figure 2: Laser disruption of cell formation in the lateral ectodermal region. (a) Cross-section and en face views showing the basal location of the “burn marks” after laser disruption in the lateral ectodermal region. No burn marks are observed at the level of the vitelline membrane. Blue and red curves in the cross-section views indicate the vitelline membrane and the position where the projections were made for the en face views. Magenta arrows: burn marks. (b) Figure 5a from Rauzi et al., 2015, clear bright burn marks can be seen from the apical surface view. (c) Overlay of the signal at T = -10 min and 0 min (onset of gastrulation) showing the movement of burn marks before gastrulation (yellow arrows).
(IV) Some panels show two distinct molecules tagged with the same or spectrally overlapping flurophores, that unfortunately localize in similar spatial patterns. This encumbers data validation.
We agree with the reviewer that having two distinct proteins tagged with the same fluorophore is not ideal for understanding the behavior of the tagged proteins, however, it usually does not affect the evaluation of the cell or tissue morphology, as far as the cell membrane is explicitly labeled. For example, in our original Figure 2 (new Figure 4), although GFP is tagged on both CIBN and Sqh, and mCherry is tagged on both CRY2-Rho1DN and Sqh, the cell and tissue morphology is clearly discernable by these markers, which allowed us to evaluate the progression of ventral furrow formation. In the cases where there was a need to evaluate the behavior of a particular molecule (e.g. Sph), we always repeated the experiments in a way such that the molecule of interest is tagged with a distinct fluorophore that does not spectrally overlap with other fluorophores – this often requires the use of an plasma membrane anchored CIBN that is not fluorescently tagged (e.g. Figure 1, Figure 4 – figure supplement 3).
(V) The physical model is a central part for data interpretation. In its current form it is very challenging to follow. It is also critical the system be studied with proper cell aspect ratio, as the elasticity of thin sheets has a well established non-linear thickness dependence.
These are valid critiques of our thin layer physical model (original Figure 5). The original purpose of this model is not to recapitulate the actual furrow morphology or cell shape change observed in the actual embryo, but rather to test the possibility of recapitulating the acceleration in tissue flow during the folding process by combining local constriction and global compression in a spherical (circular in 2D) elastic shell. Developing a dynamic vertex model that contains the realistic cell aspect ratio comparable to the actual cells in the embryo while displaying realistic cellular dynamics during the folding process is nontrivial and need substantial further development of the model. Since the manuscript is now focused on the bistable characteristics of the mesoderm during gastrulation rather than tissue dynamics during the folding process, we decide to leave the dynamics vertex model out of the revised manuscript, as suggested by the editor.
Reviewer #2 (Public Review):
Guo and colleagues aim to unravel the mechanisms driving the fast process of mesoderm invagination in the Drosophila early developing embryo. While cell apical constriction is known to drive ventral furrowing (1st phase), it is still not clear if apical constriction is necessary/sufficient to drive mesoderm internalization (2nd phase) and weather other mechanisms cooperate during this process. By using 1ph optogenetics, the authors cannot test specifically the role of apical constriction but can systematically affect the overall actomyosin network in ventral cells in a time specific fashion (1-minute resolution). In this way, they come to the conclusion that actomyosin contractility is necessary for the 1st phase but not for the 2nd phase of mesoderm invagination. Interestingly, they conclude that the system is bistable. In the second part of this study, the authors test the role of the coupling between mesoderm and ectoderm by using 2D computational modelling and infrared pulsed laser dissection. They propose that the ectoderm can generate compressive forces on the mesoderm facilitating mesoderm internalization (2nd phase).
This project is of interest since it tackles a key morphogenetic process that is necessary for the development of the embryo. The conclusion of 'bistability' resulting from the RhoDN optogenetic experiments (1st part of this study) are well supported and quite interesting. The IR laser experiments used to tackle the coupling between ectoderm and mesoderm (2nd part of the study) are key to support main conclusions, nevertheless their experimental design and results are puzzling. It is not clear what the authors are actually doing to the tissues. The experiments performed in the 2nd part of this study need to be revisited and conclusions eventually softened.
Major comments:
- The 920 nm laser ablation of ectoderm cells is a key experiment in this study to support the ectoderm compression hypothesis. Nevertheless, this experiment is puzzling: the rationale of the experimental design, the effect of the laser on cells and the interpretation of the results are unclear.
The rationale for the laser ablation experiment designed to test tissue compression is analogous to the widely used laser ablation approach for detecting tissue tension (Rauzi et al., 2015; Rauzi et al., 2008). In typical experiments where laser ablation was used to measure tensile stresses in cells and tissues, ablation of cells or subcellular structures that are under tension results in recoil of surrounding cell/tissue structures. We reasoned that if the tissue is under compression, similar laser treatment should result in shrinking of the laser-treated region, as the cells in the laser-treated region are expected to have an impaired ability to resist compressive stresses from outside of the region.
In our experiment, we used the reduction of the width of the laser treated region within the first 10 sec after laser treatment as the measure for tissue shrinking, which we considered as an indication for the presence of compressive stresses. This tissue response, albeit mild, is not a non-specific tissue response to our laser treatment – we did not observe tissue shrinking when we treat the tissue during cellularization or within the first five minutes of gastrulation, although identical experimental conditions were used. The rate and magnitude of tissue shrinking after laser treatment is determined by multiple factors, including the level of compressive stresses, the difference in cell rigidity before and after laser treatment, and the overall viscosity of the tissue. We acknowledge that the knowledge on these factors is largely lacking, and therefore additional independent validations of our approach are needed to further strengthen our conclusion on the presence of tissue compression. Following the editor’s suggestion, we decided to remove the laser ablation experiment from the current manuscript and focus on the characterization of the optogenetic tool and the binary tissue response after acute actomyosin inhibition.
- The authors propose to use again 920 nm laser ablation but this time to "physically separate" the two ectoderms from the ventral tissue. This is again a key experiment, but it raises some concerns:
a. "Physical separation" would need to be demonstrated (e.g., EM after laser ablation). From Fig. 6b it is clear that IR laser ablation results in prominent auto-fluorescent zones. This has been already reported in previous work (De Medeiros G. et al. Scientifc Reports 2020) showing that high power and sustained IR fs laser targeting produces auto-fluorescence and highly electron-dense structures in the early developing Drosophila embryo. This process is referred to laser cauterization that does not induce separation between tissues. This structures eventually displace together with the lateral tissue (also shown in Fig.6 b). b. This strong laser "treatment", that should be ectoderm specific, results in perturbation of other non-ectoderm related processes (e.g., mesoderm apical constriction as shown by the authors). This can support the idea that many other processes are affected and that in general this laser heating "treatment" has global effects. These results might invalidate the conclusion proposed by the authors.
These are both valid critiques. As for the reviewer’s point “a”, we agree with the reviewer that a “physical separation” of the mesoderm from the ectoderm has not been rigorously demonstrated in our original manuscript. As detailed in our response to reviewer #1 comment #3, since the purpose of this experiment is to ask whether the presence of the lateral ectoderm is important for the mesoderm to transition between apical constriction and invagination (and our result shows yes), whether the approach we used physically separated the mesoderm and the ectoderm is no longer an immediately relevant question. We apologize for the vague use of “physical separation” in our original manuscript and we thank the reviewer for pointing this out.
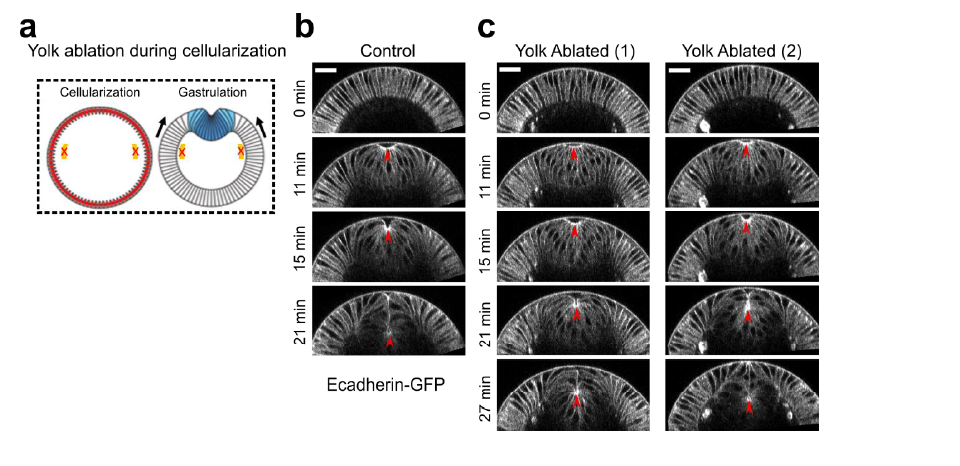
To address the reviewer’s point “b” and to ask whether the laser treatment used in our experiment has a global effect, we performed a control experiment where we treated the yolk region of the embryo with the identical approach. Despite the appearance of burn marks in the treated yolk region, mesoderm invagination proceeded largely normally under this condition, with a mild reduction in the rate of furrow invagination (Authors’ Response Figure 3). Therefore, the prominent delay in the transitional state we observed after disruption of lateral ectoderm (Original Figure 6) is not likely caused by non-specific laser heating effect. In addition, in both the yolk-ablation and the ectoderm-ablation experiments, cellularization occurred normally outside of the laser-treated regions, in further support of the lack of strong non-specific effect from our laser treatment. That being said, we acknowledge that we do not fully understand the impact of the laser treatment on the embryo (e.g., what causes the reduced rate of apical constriction), and more control experiments are required in order to fully describe the tissue response we observed. As suggested by the editor, we decided to remove the ectoderm-ablation experiment from the revised manuscript and focus on the characterization of the optogenetic tool and the binary tissue response after acute actomyosin inhibition.
Authors’ Response Figure 3. Laser treatment in the yolk region of the embryo. (a) Cartoon depicting the position of laser treatment. Similar laser condition was used as described in the original Figure 6. Laser ablation was performed during cellularization and the treated embryo was imaged during gastrulation. (b) An example control embryo without laser treatment. (d-e) Two examples showing ventral furrow formation after laser treatment in the yolk region. Only a mild delay in furrow invagination was observed. Red arrowheads indicate the invagination front. Scale bar: 25μm.
Reviewer #3 (Public Review):
The authors address how contractile forces near the apical surface of a cell sheet drive out-of-plane bending of the sheet. To determine whether actomyosin contractility is required throughout the folding process and to identify potential actomyosin independent contributions for invagination, they develop an optogenetic-mediated inhibition of myosin and show that myosin contractility is critical to prevent tissue relaxation during the early stage of folding but is dispensable for the deepening of the invagination. Their results support the idea that the mesoderm is mechanically bistable during gastrulation. They propose that this mechanical bistability arises from an in-plane compression from the surrounding ectoderm and that mesoderm invagination is achieved through the combination of apical constriction and tissue compression. Regarding global message of the manuscript, I have two main critics. The authors consider their work as the first to prove that there is a additional mechanism to apical constriction leading to invagination. This is not true. First, the fact that the ectoderm could exert a compressive force on the invaginating mesoderm is not new and has been not only proposed, but tested previously (Rauzi and Leptin, 2015). Second, several recent publications demonstrated that on top of apical constriction, lateral forces were also required for the invagination and the authors ignore these data (Gracia et al, 2019 ; John et al, 2021).
We thank the reviewer for this important comment. In the original Introduction, we have mentioned several previous studies that suggest the presence of additional mechanisms to apical constriction during ventral furrow formation. We stated: “The observation that the maximal rate of apical constriction and the maximal rate of tissue invagination occur at distinct times suggests that apical constriction does not directly cause tissue invagination (Polyakov et al., 2014; Rauzi et al., 2015). A number of computational models also predict that mesoderm invagination requires additional mechanical input, such as “pushing” forces from the surrounding ectodermal tissues, but experimental evidence for this additional mechanical input remains sparse (Munoz et al., 2007; Conte et al., 2009; Allena et al., 2010; Brodland et al., 2010).”
To address the reviewer’s comment, in the revised manuscript, we expanded this paragraph to further elaborate the previous contributions: “However, accumulating evidence suggests that apical constriction does not directly drive invagination during the shortening phase. First, it has been observed that the maximal rate of apical constriction (or cell lengthening) and the maximal rate of tissue invagination occur at distinct times (Polyakov et al., 2014; Rauzi et al., 2015). Second, it has been previously proposed, and more recently experimentally demonstrated, that myosin accumulated at the lateral membranes of constricting cells (‘lateral myosin’) facilitates furrow invagination by exerting tension along the apical-basal axis of the cell (Brodland et al., 2010; Conte et al., 2012; Gracia et al., 2019; John and Rauzi, 2021). Finally, a number of computational models predict that mesoderm invagination requires additional mechanical input from outside of the mesoderm, such as “pushing” forces from the surrounding ectodermal tissue (Munoz et al., 2007; Conte et al., 2009; Allena et al., 2010; Brodland et al., 2010). These models are in line with the finding that blocking the movement of the lateral ectoderm by laser cauterization inhibits mesoderm invagination (Rauzi et al., 2015). A similar disruption of ventral furrow formation can also be achieved by increasing actomyosin contractility in the lateral ectoderm (Perez-Mockus et al., 2017). While these pioneer studies highlight the importance of cross-tissue coordination during mesoderm invagination, the actual mechanical mechanism that drives the folding of the mesodermal epithelium and the potential role of the surrounding ectodermal tissue remain to be elucidated.”
One of the motivations for us to develop experimental approaches to detect compression in the ectoderm (original Figure 4) and to disrupt the ectoderm (original Figure 6) is the lack of direct evidence demonstrating the mechanical contribution of the ectoderm to mesoderm invagination. Several studies have shown that manipulations of the ectodermal tissue can impair ventral furrow formation. One study shows that preventing the movement of the lateral ectoderm, by anchoring ectodermal cell apices to the vitelline membrane, blocks ventral furrow invagination(Rauzi et al., 2015). Another study shows that upregulation of apical myosin contractility in the lateral ectodermal tissues can inhibit or even reverse the furrow invagination process (Perez-Mockus et al., 2017). These results indicate that an increase in the resistance to mesoderm movement can impair mesoderm invagination. However, this would be expected even if the ectoderm does not provide active mechanical input to facilitate mesoderm invagination. Therefore, these experiments, while very informative, did not provide direct evidence for a role of ectodermal compression in mesoderm invagination.
Another motivation for us to examine potential mechanisms outside of the mesoderm is the observation that ventral furrow invagination continues even when both apical myosin and lateral myosin are disrupted after Ttrans (Late Group embryos). This result indicates that factors other than apical or lateral myosin must be responsible for the invagination of the furrow in Late Group embryos. In the revised manuscript, we used a modeling approach to demonstrate that lateral myosin and ectodermal compression may function in parallel to promote the invagination of the ventral furrow (Figure 7). In the revised Discussion, we propose that “ventral furrow formation is mediated through a joint action of multiple mechanical inputs. Apical constriction drives initial indentation of ventral furrow, which primes the tissue for folding, whereas the subsequent rapid folding of the furrow is promoted by bistable characteristic of the mesoderm and by lateral myosin contractions in the constricting cells.”
They generated an optogenetic tool, "Opto-Rho1DN", to inhibit Rho1 through light-dependent plasma membrane recruitment of a dominant negative form of Rho1 (Rho1DN). The specificity of local inactivation of Myosin was tested on apical myosin before and during invagination. They observed a strong reduction of Myosin II recruitment and a phenotype that mimicks Rok inhibition. They found that acute loss of myosin contractility during most of the lengthening phase results in immediate relaxation of the constricted tissue, but similar treatment near or after the lengthening-shortening transition does not impede invagination. They conclude that the second part of furrow invagination is not due to myosin activities at the apical or lateral cortices of the mesodermal cells and that actomyosin contractility is required in the early but not the late phase of furrow formation. This part regarding the temporal requirement of Myosin during invagination brings novelty in the field since it has never been tested before.
We thank the reviewer for the comment on the novelty of our work.
They observe that ectodermal cells shorten their apico-basal axis prior to Ttrans, and that compression from the ectoderm is independent of ventral furrow formation since it still occurs even if invagination is inhibited.
They further develop two types of simulations to test theoretically the importance of compressive stress in the invagination process. The theoretical part would need to be further developed and discussed. They would need to integrate all the different components that have been shown to be essential for the invagination (not only apical constriction) and the dynamic aspect of the vertex model has to be clearly explained.
We thank the reviewer for the suggestions on the modeling parts. In the energy-based vertex model (the Polyakov model, original Figure 3), two previously identified mechanisms, apical constriction and basal relaxation, have been implemented in the model to drive lengthening-shortening cell shape change and furrow invagination. Following the reviewer’s suggestions, we have modified the Polyakov model to include additional mechanisms that have been shown to facilitate ventral furrow invagination. In particular, we focused our analysis on the role of lateral myosin in the constricting cells on furrow invagination (Figure 7). Please refer to our response to the combined comments for details (in the section “ Additional modeling analysis to test the known mechanisms for mesoderm invagination”).
As for the dynamic vertex model presented in our original manuscript (original Figure 5), as detailed in our response to Reviewer #1’s comment #5, since the revised manuscript is focused on the bistable characteristics of the mesoderm during gastrulation rather than tissue dynamics during the folding process, we decide to leave this part out of our revised manuscript as suggested by the editor.
-

Evaluation Summary:
This manuscript studies a topic of interest to developmental biologists using a combination of optogenetics, biophysical concepts, and mathematical modeling. How in plane contractile forces cause out of plane shape changes is a relevant open question, and the optogenetic tools developed in this work provide a new strategy to address the question.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This work raises the question of how in plane forces generated at the apical surface of an epithelial cell sheet cause out of plane motion, an important morphogenetic motif. To address this question, a new ontogenetic dominant negative rho1 tool, based on the cry2-CIBN system is presented. The authors use this tool to analyze the well studied biophysical process of ventral furrow formation, and dissect the spatiotemporal requirement of rho1 signaling to modulate myosin accumulation. They separate the effect on morphogenesis into an early phase that becomes significantly slowed down by myosin inhibition, and a late phase where the kinetics is comparable to wild type despite treatment. For interpretation of the data, an older model of cell mechanics treating tissue as a purely elastic material is presented. It …
Reviewer #1 (Public Review):
This work raises the question of how in plane forces generated at the apical surface of an epithelial cell sheet cause out of plane motion, an important morphogenetic motif. To address this question, a new ontogenetic dominant negative rho1 tool, based on the cry2-CIBN system is presented. The authors use this tool to analyze the well studied biophysical process of ventral furrow formation, and dissect the spatiotemporal requirement of rho1 signaling to modulate myosin accumulation. They separate the effect on morphogenesis into an early phase that becomes significantly slowed down by myosin inhibition, and a late phase where the kinetics is comparable to wild type despite treatment. For interpretation of the data, an older model of cell mechanics treating tissue as a purely elastic material is presented. It fails to reproduce the observations. As a modification, in analogy to buckling of a thin beam under load, a compressive stress exerted by the adjacent ectoderm is introduced. Further analysis of cell behaviors in response to various laser mediated tissue manipulations is presented as support of the proposed mechanism.
Overall, the manuscript addresses an important aspect of morphogenesis. In particular the use of optogenetic tools promises new insights that might be more challenging to achieve with traditional mutant analysis. However, reservations remain with respect to (1) rigor of the analysis, and (2) interpretation and quality of the data in support of the proposed mechanism; this applies in particular to presentation of biophysical observations, including experiment and simulations.
The manuscript adds valuable quantitative data, in particular the findings described in Fig 2ab. However, insufficient analysis are performed to fully support the claims of the manuscript by the data presented.
(I) The manuscript proposes an elasticity based model of tissue mechanics, but provides no experimental evidence in support of this assumption. Many rheology studies performed in a wide range of specimen (including the Drosophila embryo) found a separation of time scales, that shows elasticity is a good approximation of tissue mechanics only for time scales short compared to the process studied here.
(II) The manuscript uses a method of micro-dissection to soften cells, but does not provide a clear definition of the concept softening, provides no rational for the methods functioning, and does not provide independent validation. The described treatment might affect cells in many alternative ways to the offered interpretation. This data is the central experimental evidence given in support of the proposed ectoderm compression mechanism, and therefore it is essential to provide a precise physical explanation of the method, and validation of measurements that bolster the conclusion.
(III) Mechanical isolation of the mesoderm is a very exciting approach to test the possible involvement of adjacent tissues in folding. Indeed, the authors report a delay of ventral furrow formation. However, there is no evidence provided that (a) the mesoderm is mechanically uncoupled, and (b) that the treatment did not have undesired side effects. For example, a similar procedure (so-called cauterization, see Rauzi 2015) has been used to immobilize cells in the Drosophila embryo. Such an effect could account for the observed delay in furrow formation.
(IV) Some panels show two distinct molecules tagged with the same or spectrally overlapping flurophores, that unfortunately localize in similar spatial patterns. This encumbers data validation.
(V) The physical model is a central part for data interpretation. In its current form it is very challenging to follow. It is also critical the system be studied with proper cell aspect ratio, as the elasticity of thin sheets has a well established non-linear thickness dependence. -

Reviewer #2 (Public Review):
Guo and colleagues aim to unravel the mechanisms driving the fast process of mesoderm invagination in the Drosophila early developing embryo. While cell apical constriction is known to drive ventral furrowing (1st phase), it is still not clear if apical constriction is necessary/sufficient to drive mesoderm internalization (2nd phase) and weather other mechanisms cooperate during this process. By using 1ph optogenetics, the authors cannot test specifically the role of apical constriction but can systematically affect the overall actomyosin network in ventral cells in a time specific fashion (1-minute resolution). In this way, they come to the conclusion that actomyosin contractility is necessary for the 1st phase but not for the 2nd phase of mesoderm invagination. Interestingly, they conclude that the system …
Reviewer #2 (Public Review):
Guo and colleagues aim to unravel the mechanisms driving the fast process of mesoderm invagination in the Drosophila early developing embryo. While cell apical constriction is known to drive ventral furrowing (1st phase), it is still not clear if apical constriction is necessary/sufficient to drive mesoderm internalization (2nd phase) and weather other mechanisms cooperate during this process. By using 1ph optogenetics, the authors cannot test specifically the role of apical constriction but can systematically affect the overall actomyosin network in ventral cells in a time specific fashion (1-minute resolution). In this way, they come to the conclusion that actomyosin contractility is necessary for the 1st phase but not for the 2nd phase of mesoderm invagination. Interestingly, they conclude that the system is bistable. In the second part of this study, the authors test the role of the coupling between mesoderm and ectoderm by using 2D computational modelling and infrared pulsed laser dissection. They propose that the ectoderm can generate compressive forces on the mesoderm facilitating mesoderm internalization (2nd phase).
This project is of interest since it tackles a key morphogenetic process that is necessary for the development of the embryo. The conclusion of 'bistability' resulting from the RhoDN optogenetic experiments (1st part of this study) are well supported and quite interesting. The IR laser experiments used to tackle the coupling between ectoderm and mesoderm (2nd part of the study) are key to support main conclusions, nevertheless their experimental design and results are puzzling. It is not clear what the authors are actually doing to the tissues. The experiments performed in the 2nd part of this study need to be revisited and conclusions eventually softened.
Major comments:
The 920 nm laser ablation of ectoderm cells is a key experiment in this study to support the ectoderm compression hypothesis. Nevertheless, this experiment is puzzling: the rationale of the experimental design, the effect of the laser on cells and the interpretation of the results are unclear.
The authors propose to use again 920 nm laser ablation but this time to "physically separate" the two ectoderms from the ventral tissue. This is again a key experiment, but it raises some concerns:
a. "Physical separation" would need to be demonstrated (e.g., EM after laser ablation). From Fig. 6b it is clear that IR laser ablation results in prominent auto-fluorescent zones. This has been already reported in previous work (De Medeiros G. et al. Scientifc Reports 2020) showing that high power and sustained IR fs laser targeting produces auto-fluorescence and highly electron-dense structures in the early developing Drosophila embryo. This process is referred to laser cauterization that does not induce separation between tissues. This structures eventually displace together with the lateral tissue (also shown in Fig.6 b).
b. This strong laser "treatment", that should be ectoderm specific, results in perturbation of other non-ectoderm related processes (e.g., mesoderm apical constriction as shown by the authors). This can support the idea that many other processes are affected and that in general this laser heating "treatment" has global effects. These results might invalidate the conclusion proposed by the authors. -

Reviewer #3 (Public Review):
The authors address how contractile forces near the apical surface of a cell sheet drive out-of-plane bending of the sheet. To determine whether actomyosin contractility is required throughout the folding process and to identify potential actomyosin independent contributions for invagination, they develop an optogenetic-mediated inhibition of myosin and show that myosin contractility is critical to prevent tissue relaxation during the early stage of folding but is dispensable for the deepening of the invagination. Their results support the idea that the mesoderm is mechanically bistable during gastrulation. They propose that this mechanical bistability arises from an in-plane compression from the surrounding ectoderm and that mesoderm invagination is achieved through the combination of apical constriction …
Reviewer #3 (Public Review):
The authors address how contractile forces near the apical surface of a cell sheet drive out-of-plane bending of the sheet. To determine whether actomyosin contractility is required throughout the folding process and to identify potential actomyosin independent contributions for invagination, they develop an optogenetic-mediated inhibition of myosin and show that myosin contractility is critical to prevent tissue relaxation during the early stage of folding but is dispensable for the deepening of the invagination. Their results support the idea that the mesoderm is mechanically bistable during gastrulation. They propose that this mechanical bistability arises from an in-plane compression from the surrounding ectoderm and that mesoderm invagination is achieved through the combination of apical constriction and tissue compression.
Regarding global message of the manuscript, I have two main critics. The authors consider their work as the first to prove that there is a additional mechanism to apical constriction leading to invagination. This is not true.
First, the fact that the ectoderm could exert an compressive force on the invaginating mesoderm is not new and has been not only proposed, but tested previously (Rauzi and Leptin, 2015). Second, several recent publications demonstrated that on top of apical constriction, lateral forces were also required for the invagination and the authors ignore these data (Gracia et al, 2019 ; John et al, 2021).
They generated an optogenetic tool, "Opto-Rho1DN", to inhibit Rho1 through light-dependent plasma membrane recruitment of a dominant negative form of Rho1 (Rho1DN).
The specificity of local inactivation of Myosin was tested on apical myosin before and during invagination. They observed a strong reduction of Myosin II recruitment and a phenotype that mimicks Rok inhibition. They found that acute loss of myosin contractility during most of the lengthening phase results in immediate relaxation of the constricted tissue, but similar treatment near or after the lengthening-shortening transition does not impede invagination.
They conclude that the second part of furrow invagination is not due to myosin activities at the apical or lateral cortices of the mesodermal cells and that actomyosin contractility is required in the early but not the late phase of furrow formation.
This part regarding the temporal requirement of Myosin during invagination brings novelty in the field since it has never been tested before.They observe that ectodermal cells shorten their apico-basal axis prior to Ttrans, and that compression from the ectoderm is independent of ventral furrow formation since it still occurs even if invagination is inhibited.
They further develop two types of simulations to test theoretically the importance of compressive stress in the invagination process.
The theoretical part would need to be further developed and discussed. They would need to integrate all the different components that have been shown to be essential for the invagination (not only apical constriction) and the dynamic aspect of the vertex model has to be clearly explained.
-