Handling of intracellular K + determines voltage dependence of plasmalemmal monoamine transporter function
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This paper will be of interest to scientists interested in mechanistic studies of ion-coupled transporters. The authors demonstrate that dopamine, catecholamine and serotonin transporters - albeit structurally very similar - differ in the number of transport substrates and they define the underlying functional basis of this difference using a range of sophisticated techniques. This is an extremely nice and interesting study. providing new tools and new insights into an important class of transporter. Since many drugs that block one of the transporters also modify the two others, the paper may help to define pharmaceutical approaches that specifically block only one of them and that might allow for a better treatment of psychiatric diseases. The data analysis is rigorous and the conclusions are justified by the data, but the paper should be made more "user friendly" so that a wider audience could appreciate it better.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The concentrative power of the transporters for dopamine (DAT), norepinephrine (NET) and serotonin (SERT) is thought to be fueled by the transmembrane Na + gradient, but it is conceivable that they can also tap other energy sources, e.g. membrane voltage and/or the transmembrane K + gradient. We address this by recording uptake of endogenous substrates or the fluorescent substrate APP + ((4-(4-dimethylamino)phenyl-1-methylpyridinium) under voltage control in cells expressing DAT, NET or SERT. We show that DAT and NET differ from SERT in intracellular handling of K + . In DAT and NET, substrate uptake was voltage-dependent due to the transient nature of intracellular K + binding, which precluded K + antiport. SERT, however, antiports K + and achieves voltage-independent transport. Thus, there is a trade-off between maintaining constant uptake and harvesting membrane potential for concentrative power, which we conclude to occur due to subtle differences in the kinetics of co-substrate ion binding in closely related transporters.
Article activity feed
-

Author Response:
Reviewer #1:
This work is aiming at the characterization of the molecular and kinetic mechanism of how three members of the SLC6 family of transporters, namely for dopamine (DAT), norepinephrine (NET), and serotonin (SERT), transport substrate across the membrane, and how the transport process is affected by cations. The authors use electrophysiology and sophisticated rapid solution exchange methods, in conjunction with fluorescence recordings from single cells, to correlate flux (from fluorescence) with electrical activity (from currents).
The strength of the methods is based on the application of a kinetic method with high time resolution, allowing the isolation of fast processes in the transport mechanism, and their modeling using a kinetic multistep scheme. In particular useful is the combination with …
Author Response:
Reviewer #1:
This work is aiming at the characterization of the molecular and kinetic mechanism of how three members of the SLC6 family of transporters, namely for dopamine (DAT), norepinephrine (NET), and serotonin (SERT), transport substrate across the membrane, and how the transport process is affected by cations. The authors use electrophysiology and sophisticated rapid solution exchange methods, in conjunction with fluorescence recordings from single cells, to correlate flux (from fluorescence) with electrical activity (from currents).
The strength of the methods is based on the application of a kinetic method with high time resolution, allowing the isolation of fast processes in the transport mechanism, and their modeling using a kinetic multistep scheme. In particular useful is the combination with fluorescence recording from single cells, which allows the authors to measure flux and current in the same cell under voltage clamp conditions. This is an elegant approach to get information on the voltage dependence of substrate flux, which is difficult to obtain with other methods. As to the strength of the results, the data are generally of high quality, showing the kinetic and mechanistic similarities and differences between the three transporters under observation. Another strength is that the results are quantitatively represented by kinetic simulations, which appear to fit the experimental data well.
The major weakness of the research is related to interpretation of the experimental results. While the authors propose a unified K+ interaction mechanism for the three transporters, DAT, NET and SERT, the proposed K+ association/dissociation mechanism is 1) highly unusual, and 2) not unique in the ability to explain the experimental data. As to point 1), the DAT mechanism (Fig. 7A) proposes a sequence of intracellular K+ association and dissociation steps. Since the intracellular [K+] remains constant, such a sequence requires a change of affinity for K+, which is initially high when K+ associates (33 microM according to the provided rate constants) and then has to be low for K+ dissociation (3.3 mM). Such an affinity change requires input of free energy, to promote K+ dissociation. From the provided rate constants and at room temperature this free energy change can be approximated as 11.4 kJ/mol. This is a large energy amount, in fact larger than what is stored in the physiological concentration gradient for one Na+ ion as a driving force for transport. It appears that the transporter would waste a lot of energy for no apparent benefit, with a futile K+ association/dissociation cycle, that would just generate heat.
Therefore, while the authors have achieved their aim of quantitatively assessing transporter function and thorough description by a kinetic mechanism, their final proposed mechanism does not support all of the conclusions because it is by far from unique in being able to explain the data (point 2) above). While this may be true for other transport mechanisms proposed in the past, the mechanism proposed here is somewhat odd with respect to energy requirements. Thus, it would require extraordinary experimental proof to propose it in exclusion of other, maybe more plausible mechanisms.
Despite these shortcomings, the potential impact of the work is high, because a unifying theory of cation interaction and stoichiometry of the monoamine transporter members of the SLC6 family has been missing in the literature. In addition, the elegant method of combining single cell electrophysiology and fluorescence flux measurements is impactful, especially in the whole cell recording method, allowing the control of intracellular ionic composition.
We thank reviewer 1 for his comments on the kinetic modelling. We do not claim that the mechanism, which we propose, is unique in its ability to explain the data. However, we should like to argue that the proposed mechanism is plausible and parsimonious. We, much like reviewer 1, initially asked the question, whether a mechanism requiring an ion such as potassium to associate and subsequently dissociate from the same side of the transporter was energetically feasible. In fact, one of the main reasons for employing kinetic models was to address this specific issue.
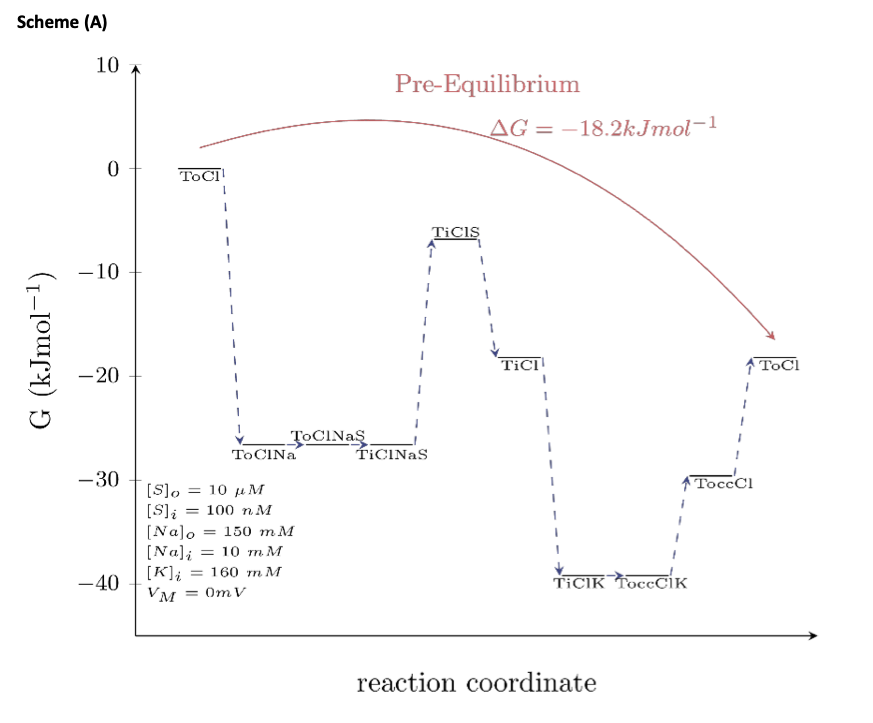
If detailed balance in a kinetic model is maintained (i.e., the product of the rates in the forward direction of a loop equals the product of the rates in the reverse direction), the model is energetically sound (i.e., such a model does not violate the laws of thermodynamics). It is true that for a spontaneous reaction to occur, the Gibbs free energy has to be negative. In a multistep process, however, this consideration only pertains to the “initial” and the “final” state. As long as the Gibbs free energy between these two states is negative the reaction will proceed, even if the Gibbs free energy between “intermediate” states is positive. This point is illustrated in the schemes below.
Scheme (A) maps out the Gibbs free energy of the outer loop of the kinetic model of DAT (i.e., this path describes the conformational trajectory, which the transporter takes in the presence of intracellular K+- see scheme in Fig.7A of the manuscript). For calculating the Gibbs free energy of this loop, we assumed a pre-equilibrium condition (i.e., an extracellular and intracellular substrate concentration that we arbitrarily set to 10 μM and 100 nM, respectively) and the membrane voltage as 0 mV. As shown in the scheme, the Gibbs free energy between the “initial-left” and the “final-right” state is negative. Accordingly, the multistep reaction can proceed spontaneously.
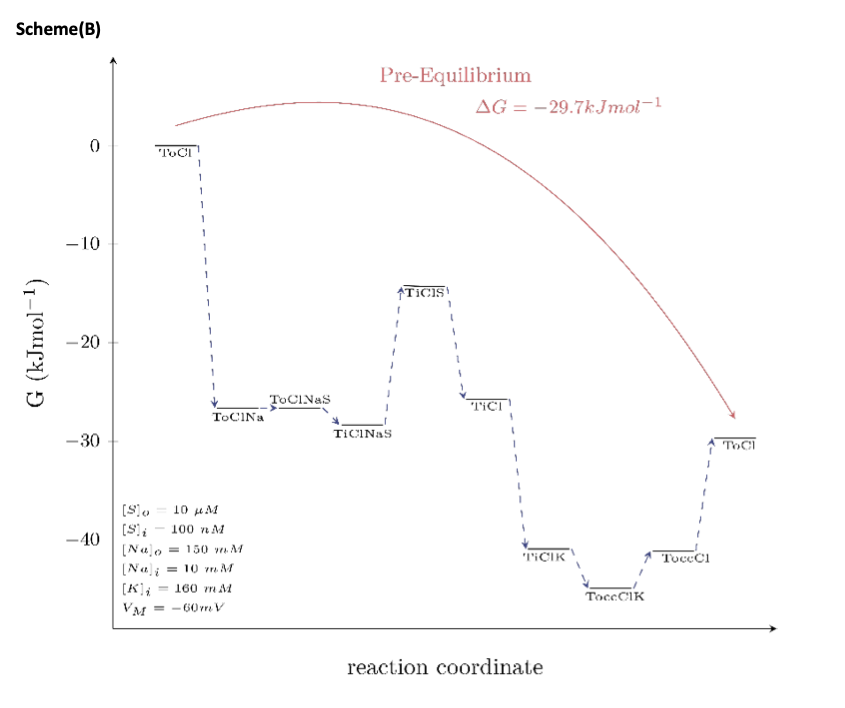
In scheme (B), we mapped out the Gibbs free energy for the same path and the same pre-equilibrium condition as shown in scheme (A); the only difference is that the membrane potential was now assumed to be -60 mV. This is to show that voltage is also a determining factor of the extent by which the Gibbs free energy changes.
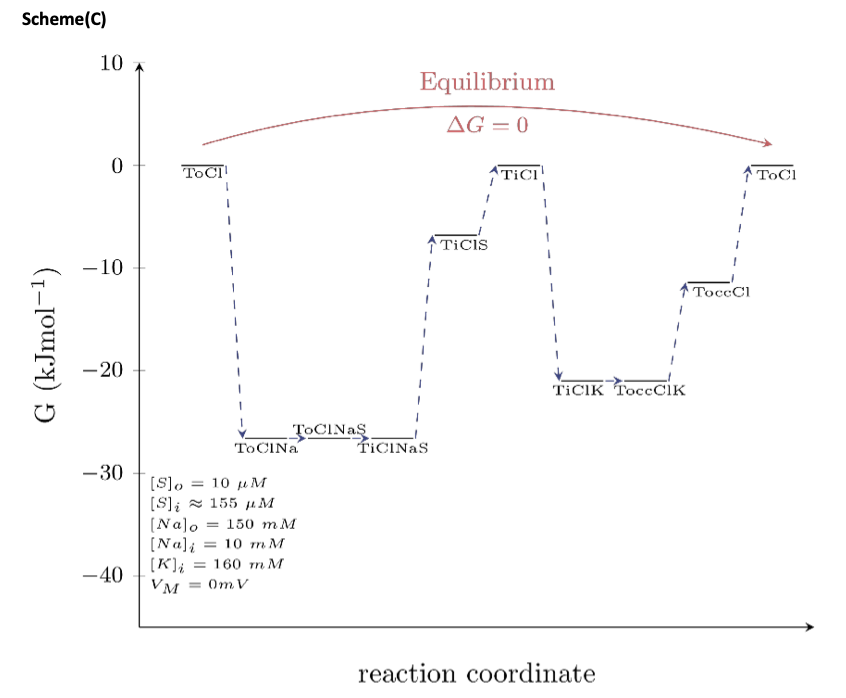
In Scheme (C), we mapped out the Gibbs free energy at equilibrium (the difference in Gibbs free energy between the “initial” and the “final” state is zero). This condition is met when the intracellular substrate concentration is 155 μM. At this intracellular substrate concentration, the energy stored in the substrate gradient notably matches exactly the energy of the Na+ gradient. The model therefore predicts that no energy is dissipated as heat, an observation that is in contrast to the concern raised by reviewer 1. We admit that the model can be criticized on this ground, because arguably, a realistic process is expected to dissipate energy as heat even if it involves a microscopic system (as is the case here). Determination of how much heat is generated in a transport cycle is, however, beyond the scope of the present manuscript and warrants a detailed study. In such a study, one could investigate if any heat loss generated can be compensated by, for instance, the occasional antiport of K+ by DAT, which, as we point out in the discussion, is possible. In this context, we stress that the energetic costs would have been much higher, if we had assumed non-obligatory antiport of K+ through DAT. Such a mechanism predicts that the K+ gradient is constantly dissipated in the absence of the substrate, which would indeed create the futile heat loss reviewer 1 is concerned about.
An alternate hypothesis to the actions of intracellular K+ on the DAT transport cycle would be to propose the presence of a regulatory K+ binding site. We are reluctant to assume this mechanism for the simple reason that there is little evidence for such sites from the available crystal structures. The view that K+ binds to Na2 site in DAT, NET and SERT is consistent with our data (see Fig.5). These observations are aided by a previous study that shows K+ can bind to the Na2 site in DAT, as determined by extensive molecular dynamic simulations (Razavi et al., 2017, cited in the manuscript). By its very nature, the Na2 site cannot serve as a regulatory K+ binding site; for the transporter to proceed in the transport cycle, K+ must at some point dissociate from the Na2 site.
On further scrutiny of our model for DAT, NET and SERT, we noticed that the extra and intracellular affinities for Na+ were set too high. We regret this oversight that arose because we had only simulated experiments in which the intracellular Na+ concentrations had been zero. The selected Na+ affinities would not have allowed the transporter to function properly at a physiological intracellular Na+ concentration (which is ~10 mM). We now rectified this problem by lowering the inner and outer Na+ affinity by a factor of 10. In Fig.7 of the main manuscript and supplementary figure 6, we have now replaced all previous simulations of the three transporters with the predictions of the newly amended model. As seen, the changes in the binding parameters for Na+ in the model could still account for the key findings of this study.
Reviewer #2:
Bhat et al. study transport mechanism of three members of the SLC6 family, i.e. DAT, NET and SERT, using a combination of cellular electrophysiology, fluorescence measurements - taking advantage of a fluorescent substrate (APP+) that can be transported by each of these different transporters - and kinetic modelling. They find that DAT, NET and SERT differ in intracellular K+ binding. In DAT and NET, intracellular K+ binding is transient, resulting in voltage-dependent transport. In contrast, SERT transports K+, and the addition of a charged substrate to the transport cycle makes serotonin transport voltage-independent.
This is an extremely nice and interesting manuscript, based on a series of beautifully designed and executed experiments that are convincingly analyzed via a kinetic model. I have only some suggestions:
- Fig. 4: I find the description of Fig. 4 extremely difficult to understand. In clear contrast to the introductory sentence "Previous studies showed that Kin+ was antiported by SERT, but not by NET or DAT (Rudnick & Nelson, 1978; Gu et al., 1996; Erreger et al.,2008), SERT appears to be able to transport APP+ without K+ in Fig. 4. I was trying to understand this obvious discrepancy for a long time, until I found the authors coming back to this point in the discussion "However steady-state assessment of transporter mediated substrate uptake is hindered by the fact that all three monoamine transporters can also transport substrate in the absence of Kin+". This is a little late, and the author should address this point more explicitly in the result section, close to the description of Fig. 4.
We agree with reviewer 2’s comments pertaining to the SERT data represented in Fig.4C. The observations made from this dataset seem confusing in the absence of any relevant context. We have added the following statements to clarify any discrepancy arising from Fig. 4 (lines 266-273): “Owing to the instrumental role of Kin+ in the catalytic cycle of SERT, the observed lack of difference in APP+ uptake profiles by SERT-expressing cells in the presence or absence of Kin+ seem contradictory. This discrepancy can be explained as follows: 1) SERT can alternatively antiport protons to complete its catalytic cycle (Keyes and Rudnick, 1982; Hasenhuetl et al. 2016) and 2) APP+ is a poor SERT substrate (as determined by lack of APP+ induced steady state currents, Fig. 2F and 3F) that may be shuttled into SERT-expressing cells at rates slower than the rate limiting isomerization of SERT from inward open to outward open state.”
- Throughout the whole manuscript I am missing statistical details in comparisons.
Statistical details for comparisons, which were done on some data sets in Fig. 4, Fig.5 and Fig.6, have now been incorporated in the manuscript text.
- Since APP+ might also only bind to the transporter or even only bind to the cell membrane, the authors might want to look at how the time course of the cellular APP+ signal depends on the size of the cells or on the ratio of transport currents and capacitance. It is of course possible that the tested cells do not differ sufficiently in size to permit such comparison. The authors should at least comment on this possibiliy.
We are working on monoclonal lines. Thus, the differences in cell size are small (between 25- 30 pF). In the new supplementary figure 1, we show that our (previously held) conjecture that the fast component represents membrane binding was wrong. In fact, analysis of the APP+ fluorescence in control cells (supplementary figure 1D) suggests that APP+ adherence to the plasma membrane does not contribute to significant fluorescence signal. We apologize for this misinterpretation and please refer to the responses to reviewer 1 for more details.
- Another set of results one might look at are the time courses of fluorescence decay after the end of the APP+ perfusion (Fig. 2 and 4). Substrate (APP+) outward transport should have a comparable voltage dependence as substrate uptake, moreover it should depend on the amount of substrate that entered to the cell before. Could the authors provide such result and use them to exclude specific/unspecific APP+ binding?
In supplementary figure 1 (panel, A and C) and video files 1 and 2, we show that APP+ adheres to intracellular membranes of organelles. This has also been shown previously by others (Solis Jr. et al., 2012; Karpowicz Jr et al., 2013; Wilson et al., 2014, cited in the manuscript). Because these structures serve as sinks, there is no (or only little) free APP+, which is available for outward transport.
Reviewer #3:
The sodium-coupled biogenic transporters DAT, NET and SERT, terminate the synaptic actions of dopamine, norepinephrine and serotonin, respectively. They belong to the family of Neurotransmitter:sodium:symporters. These transporters have very similar sequences and this is reflected at the structural level as judged by similarity of the crystal structures of the outward-facing conformations DAT and SERT. However, earlier functional studies indicated that transport by SERT is electroneutral because the charges sodium ions and substrate moving into the cell are compensated by the outward movement of potassium ions (or protons) to complete the transport cycle. On the other hand, DAT and NET are electrogenic. Moreover, potassium ions are not extruded by these transporters and the Authors set out to investigate if the electrogenicity is related to difference in potassium handling between SERT and the two other biogenic transporters. This was done by analyzing the role of intracellular cations and voltage on substrate transport by the three biogenic amine transporters. This was achieved by the simultaneous recording of uptake of the fluorescent substrate APP+ and the current induced by this process under voltage-clamp conditions by single HEK293 cells expressing the transporters. The Authors found that even though uptake by NET and DAT did not require internal potassium, these transporters could actually interact with internal potassium as judged by the voltage dependence of the so-called peak current. This voltage dependence was very steep in the absence of both sodium and potassium. However, in the presence of either cation this voltage dependence became less steep when either of these cations was present in the internal milieu, indicating that not only sodium but also potassium could bind from the inside. The same result was obtained with SERT. However, uptake by SERT was found to be much less dependent on the membrane voltage than that by DAT and NET and was stimulated by internal potassium, consistent with the proposed electroneutrality of the former. The observations indicate that the structural similarity of the three biogenic amine transporters is also reflected in their ability to bind potassium, even though this cation can translocate to the outside only in SERT.
Strengths:
Development of a sophisticated technique to interrogate the mechanism of sodium coupled biogenic amine transport in single cells. Rigorous analysis of the data. Conclusions supported by the data. The methodology can be used to obtain novel insights into the mechanism of other transporters.
Weaknesses:
The presentation could be made more "user friendly" by explaining in more detail what is happening as we go through the data. For instance, peak and steady state currents are shown already in Figure 1, but an (too brief) explanation is only provided when describing Figure 5. A schematic in the first part of the Results would be useful. Some information of on the structural background should be provided as well as a full description of the transport cycle, namely the number of sodium ions translocated per cycle and the argument why chloride remains bound to the transporter throughout the cycle. The control that in contrast to potassium, lithium is inert should be performed not only for DAT, but also for the two other transporters.
We thank Dr. Kanner for these recommendations. Regarding the role of Na+ and Cl- in the transport cycle of the monoamine transporters, we have briefly mentioned the same in the introduction as follows: “The crystal structure of both hSERT and dDAT show two bound Na+ ions. However, only one Na+ ion is thought to be released on the intracellular side in both transporters (Rudnick & Sandtner, 2019). Cl-, on the other hand, has been shown to play a modulatory role in the transport cycle of SERT and DAT, but Cl- is not essential for the transport stoichiometry (Erreger et al., 2008; Hasenhuetl et al., 2016).”
As for the control experiments with Li+, we are very grateful to Dr. Kanner for his suggestions. En route to extending the observations, which we obtained with DAT in the presence of high intracellular Li+, to NET and SERT, we stumbled upon some unexpected results: while IV relations of peak currents with high intracellular Li+ or NMDG+ in NET were identical (similar to DAT), SERT gave us exactly the opposite profiles. IV relations of high intracellular Li+ in SERT were as shallow as those in the presence of high +++ intracellular K or high intracellular Na . This is indicative of intracellular Li binding to SERT, an observation not previously reported that further highlights the differences in DAT/NET and SERT in cation binding. We believe that our observations with Li+ and SERT could be expanded on in a separate story. We have accordingly changed the manuscript text in the Results and Discussion as follows:
Results (lines 320-337):
“Because the absence of Kin+ affected the slope of the IV-relation of the peak current, we surmised that potassium bound from the intracellular side not only to SERT but also possibly to DAT and NET. We explored this conjecture by determining the IV relation of peak currents through all three +++ transporters in the presence of lithium (Liin = 163 mM) instead of Kin . Li is believed to be an inert cation, because it does not support substrate translocation by SLC6 transporters. As expected, the IV relation of peak currents through DAT and NET were similar in the presence of 163 mM Lin+ to those recorded in the absence of Kin+ (cf., diamond and triangle symbol in Fig. 5J and 5K). These observations clearly indicate that Kin+ binds to both DAT and NET and rule out an alternative explanation, i.e. that the effect can be accounted for water and monovalent cations briefly occupying a newly available space in the inner vestibule. SERT, on the hand, show shallow IV relations of peak currents with high Liin+ when compared to those acquired in the absence of Kin+ (cf., diamond and triangle symbol in Fig. 5L). This is indicative of Liin+ binding to SERT on the intracellular side. The exact nature of Liin+ binding to SERT has not been reported previously and warrants further investigation. The IV relations of peak currents are similar in the presence of 163 mM Kin+ (Fig. 5A-C) and of 163 mM Nain+ (Fig. 5G-I) in DAT, NET and SERT (cf. circle and square symbols in Fig. 5J-L). This is consistent with the idea that Nain+ and Kin+ bind to overlapping sites in these transporters. “
Discussion (lines 524-527):
“Interestingly, differences between DAT/NET and SERT are further substantiated by the ability of SERT+ to bind to intracellular Li . The exact nature of this interaction is unknown and necessitates an in-depth investigation that is beyond the scope of this study.”
-

Reviewer #3 (Public Review):
The sodium-coupled biogenic transporters DAT, NET and SERT, terminate the synaptic actions of dopamine, norepinephrine and serotonin, respectively. They belong to the family of Neurotransmitter:sodium:symporters. These transporters have very similar sequences and this is reflected at the structural level as judged by similarity of the crystal structures of the outward-facing conformations DAT and SERT. However, earlier functional studies indicated that transport by SERT is electroneutral because the charges sodium ions and substrate moving into the cell are compensated by the outward movement of potassium ions (or protons) to complete the transport cycle. On the other hand, DAT and NET are electrogenic. Moreover, potassium ions are not extruded by these transporters and the Authors set out to investigate if …
Reviewer #3 (Public Review):
The sodium-coupled biogenic transporters DAT, NET and SERT, terminate the synaptic actions of dopamine, norepinephrine and serotonin, respectively. They belong to the family of Neurotransmitter:sodium:symporters. These transporters have very similar sequences and this is reflected at the structural level as judged by similarity of the crystal structures of the outward-facing conformations DAT and SERT. However, earlier functional studies indicated that transport by SERT is electroneutral because the charges sodium ions and substrate moving into the cell are compensated by the outward movement of potassium ions (or protons) to complete the transport cycle. On the other hand, DAT and NET are electrogenic. Moreover, potassium ions are not extruded by these transporters and the Authors set out to investigate if the electrogenicity is related to difference in potassium handling between SERT and the two other biogenic transporters. This was done by analyzing the role of intracellular cations and voltage on substrate transport by the three biogenic amine transporters. This was achieved by the simultaneous recording of uptake of the fluorescent substrate APP+ and the current induced by this process under voltage-clamp conditions by single HEK293 cells expressing the transporters. The Authors found that even though uptake by NET and DAT did not require internal potassium, these transporters could actually interact with internal potassium as judged by the voltage dependence of the so-called peak current. This voltage dependence was very steep in the absence of both sodium and potassium. However, in the presence of either cation this voltage dependence became less steep when either of these cations was present in the internal milieu, indicating that not only sodium but also potassium could bind from the inside. The same result was obtained with SERT. However, uptake by SERT was found to be much less dependent on the membrane voltage than that by DAT and NET and was stimulated by internal potassium, consistent with the proposed electroneutrality of the former. The observations indicate that the structural similarity of the three biogenic amine transporters is also reflected in their ability to bind potassium, even though this cation can translocate to the outside only in SERT.
Strengths:
Development of a sophisticated technique to interrogate the mechanism of sodium coupled biogenic amine transport in single cells. Rigorous analysis of the data. Conclusions supported by the data. The methodology can be used to obtain novel insights into the mechanism of other transporters.
Weaknesses:
The presentation could be made more "user friendly" by explaining in more detail what is happening as we go through the data. For instance, peak and steady state currents are shown already in Figure 1, but an (too brief) explanation is only provided when describing Figure 5. A schematic in the first part of the Results would be useful. Some information of on the structural background should be provided as well as a full description of the transport cycle, namely the number of sodium ions translocated per cycle and the argument why chloride remains bound to the transporter throughout the cycle. The control that in contrast to potassium, lithium is inert should be performed not only for DAT, but also for the two other transporters.
-

Reviewer #2 (Public Review):
Bhat et al. study transport mechanism of three members of the SLC6 family, i.e. DAT, NET and SERT, using a combination of cellular electrophysiology, fluorescence measurements - taking advantage of a fluorescent substrate (APP+) that can be transported by each of these different transporters - and kinetic modelling. They find that DAT, NET and SERT differ in intracellular K+ binding. In DAT and NET, intracellular K+ binding is transient, resulting in voltage-dependent transport. In contrast, SERT transports K+, and the addition of a charged substrate to the transport cycle makes serotonin transport voltage-independent.
This is an extremely nice and interesting manuscript, based on a series of beautifully designed and executed experiments that are convincingly analyzed via a kinetic model. I have only some …
Reviewer #2 (Public Review):
Bhat et al. study transport mechanism of three members of the SLC6 family, i.e. DAT, NET and SERT, using a combination of cellular electrophysiology, fluorescence measurements - taking advantage of a fluorescent substrate (APP+) that can be transported by each of these different transporters - and kinetic modelling. They find that DAT, NET and SERT differ in intracellular K+ binding. In DAT and NET, intracellular K+ binding is transient, resulting in voltage-dependent transport. In contrast, SERT transports K+, and the addition of a charged substrate to the transport cycle makes serotonin transport voltage-independent.
This is an extremely nice and interesting manuscript, based on a series of beautifully designed and executed experiments that are convincingly analyzed via a kinetic model. I have only some suggestions:
Fig. 4: I find the description of Fig. 4 extremely difficult to understand. In clear contrast to the introductory sentence "Previous studies showed that Kin+ was antiported by SERT, but not by NET or DAT (Rudnick & Nelson, 1978; Gu et al., 1996; Erreger et al.,2008), SERT appears to be able to transport APP+ without K+ in Fig. 4. I was trying to understand this obvious discrepancy for a long time, until I found the authors coming back to this point in the discussion "However steady-state assessment of transporter mediated substrate uptake is hindered by the fact that all three monoamine transporters can also transport substrate in the absence of Kin+". This is a little late, and the author should address this point more explicitly in the result section, close to the description of Fig. 4.
Throughout the whole manuscript I am missing statistical details in comparisons.
Since APP+ might also only bind to the transporter or even only bind to the cell membrane, the authors might want to look at how the time course of the cellular APP+ signal depends on the size of the cells or on the ratio of transport currents and capacitance. It is of course possible that the tested cells do not differ sufficiently in size to permit such comparison. The authors should at least comment on this possibiliy.
Another set of results one might look at are the time courses of fluorescence decay after the end of the APP+ perfusion (Fig. 2 and 4). Substrate (APP+) outward transport should have a comparable voltage dependence as substrate uptake, moreover it should depend on the amount of substrate that entered to the cell before. Could the authors provide such result and use them to exclude specific/unspecific APP+ binding?
-

Reviewer #1 (Public Review):
This work is aiming at the characterization of the molecular and kinetic mechanism of how three members of the SLC6 family of transporters, namely for dopamine (DAT), norepinephrine (NET), and serotonin (SERT), transport substrate across the membrane, and how the transport process is affected by cations. The authors use electrophysiology and sophisticated rapid solution exchange methods, in conjunction with fluorescence recordings from single cells, to correlate flux (from fluorescence) with electrical activity (from currents).
The strength of the methods is based on the application of a kinetic method with high time resolution, allowing the isolation of fast processes in the transport mechanism, and their modeling using a kinetic multistep scheme. In particular useful is the combination with fluorescence …
Reviewer #1 (Public Review):
This work is aiming at the characterization of the molecular and kinetic mechanism of how three members of the SLC6 family of transporters, namely for dopamine (DAT), norepinephrine (NET), and serotonin (SERT), transport substrate across the membrane, and how the transport process is affected by cations. The authors use electrophysiology and sophisticated rapid solution exchange methods, in conjunction with fluorescence recordings from single cells, to correlate flux (from fluorescence) with electrical activity (from currents).
The strength of the methods is based on the application of a kinetic method with high time resolution, allowing the isolation of fast processes in the transport mechanism, and their modeling using a kinetic multistep scheme. In particular useful is the combination with fluorescence recording from single cells, which allows the authors to measure flux and current in the same cell under voltage clamp conditions. This is an elegant approach to get information on the voltage dependence of substrate flux, which is difficult to obtain with other methods. As to the strength of the results, the data are generally of high quality, showing the kinetic and mechanistic similarities and differences between the three transporters under observation. Another strength is that the results are quantitatively represented by kinetic simulations, which appear to fit the experimental data well.
The major weakness of the research is related to interpretation of the experimental results. While the authors propose a unified K+ interaction mechanism for the three transporters, DAT, NET and SERT, the proposed K+ association/dissociation mechanism is 1) highly unusual, and 2) not unique in the ability to explain the experimental data. As to point 1), the DAT mechanism (Fig. 7A) proposes a sequence of intracellular K+ association and dissociation steps. Since the intracellular [K+] remains constant, such a sequence requires a change of affinity for K+, which is initially high when K+ associates (33 microM according to the provided rate constants) and then has to be low for K+ dissociation (3.3 mM). Such an affinity change requires input of free energy, to promote K+ dissociation. From the provided rate constants and at room temperature this free energy change can be approximated as 11.4 kJ/mol. This is a large energy amount, in fact larger than what is stored in the physiological concentration gradient for one Na+ ion as a driving force for transport. It appears that the transporter would waste a lot of energy for no apparent benefit, with a futile K+ association/dissociation cycle, that would just generate heat.
Therefore, while the authors have achieved their aim of quantitatively assessing transporter function and thorough description by a kinetic mechanism, their final proposed mechanism does not support all of the conclusions because it is by far from unique in being able to explain the data (point 2) above). While this may be true for other transport mechanisms proposed in the past, the mechanism proposed here is somewhat odd with respect to energy requirements. Thus, it would require extraordinary experimental proof to propose it in exclusion of other, maybe more plausible mechanisms.
Despite these shortcomings, the potential impact of the work is high, because a unifying theory of cation interaction and stoichiometry of the monoamine transporter members of the SLC6 family has been missing in the literature. In addition, the elegant method of combining single cell electrophysiology and fluorescence flux measurements is impactful, especially in the whole cell recording method, allowing the control of intracellular ionic composition.
-

Evaluation Summary:
This paper will be of interest to scientists interested in mechanistic studies of ion-coupled transporters. The authors demonstrate that dopamine, catecholamine and serotonin transporters - albeit structurally very similar - differ in the number of transport substrates and they define the underlying functional basis of this difference using a range of sophisticated techniques. This is an extremely nice and interesting study. providing new tools and new insights into an important class of transporter. Since many drugs that block one of the transporters also modify the two others, the paper may help to define pharmaceutical approaches that specifically block only one of them and that might allow for a better treatment of psychiatric diseases. The data analysis is rigorous and the conclusions are justified by the data, …
Evaluation Summary:
This paper will be of interest to scientists interested in mechanistic studies of ion-coupled transporters. The authors demonstrate that dopamine, catecholamine and serotonin transporters - albeit structurally very similar - differ in the number of transport substrates and they define the underlying functional basis of this difference using a range of sophisticated techniques. This is an extremely nice and interesting study. providing new tools and new insights into an important class of transporter. Since many drugs that block one of the transporters also modify the two others, the paper may help to define pharmaceutical approaches that specifically block only one of them and that might allow for a better treatment of psychiatric diseases. The data analysis is rigorous and the conclusions are justified by the data, but the paper should be made more "user friendly" so that a wider audience could appreciate it better.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-