PBN-PVT projections modulate negative affective states in mice
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This study will interest neuroscientists, in particular those interested in the neurocircuitry of emotional behaviors. Using modern neuroscience techniques, the authors demonstrate that anatomical projections from a brain stem structure called the parabrachial nucleus to the paraventricular nucleus thalamus contribute to aversive states like fear and anxiety. Overall, the study offers important details of a previously uncharacterized brain circuit, although some additional experiments are required to fully substantiate the authors' claims.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Long-lasting negative affections dampen enthusiasm for life, and dealing with negative affective states is essential for individual survival. The parabrachial nucleus (PBN) and thalamic paraventricular nucleus (PVT) are critical for modulating affective states in mice. However, the functional roles of PBN-PVT projections in modulating affective states remain elusive. Here, we show that PBN neurons send dense projection fibers to the PVT and form direct excitatory synapses with PVT neurons. Activation of the PBN-PVT pathway induces robust behaviors associated with negative affective states without affecting nociceptive behaviors. Inhibition of the PBN-PVT pathway reduces aversion-like and fear-like behaviors. Furthermore, the PVT neurons innervated by the PBN are activated by aversive stimulation, and activation of PBN-PVT projections enhances the neuronal activity of PVT neurons in response to the aversive stimulus. Consistently, activation of PVT neurons that received PBN-PVT projections induces anxiety-like behaviors. Thus, our study indicates that PBN-PVT projections modulate negative affective states in mice.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
In their manuscript entitled "PBN-PVT projection modulates negative emotions in mice", Zhu et al. combine circuit mapping techniques with behavioral manipulations to interrogate the function of anatomical projections from the parabrachial nucleus (PBN) to the paraventricular nucleus of the thalamus (PVT). The study addresses an important scientific question, since the PVT and particularly the posterior PVT is known to be mostly sensitive to aversive signals, but the neural circuit mechanisms underlying this process remain unknown. Here the authors contribute important evidence that PBN inputs to the PVT may be critical for this process. Specifically, the authors identify that the PVT receives glutamatergic projections from the PBN that promote aversive behavioral responses but do not …
Author Response:
Reviewer #1 (Public Review):
In their manuscript entitled "PBN-PVT projection modulates negative emotions in mice", Zhu et al. combine circuit mapping techniques with behavioral manipulations to interrogate the function of anatomical projections from the parabrachial nucleus (PBN) to the paraventricular nucleus of the thalamus (PVT). The study addresses an important scientific question, since the PVT and particularly the posterior PVT is known to be mostly sensitive to aversive signals, but the neural circuit mechanisms underlying this process remain unknown. Here the authors contribute important evidence that PBN inputs to the PVT may be critical for this process. Specifically, the authors identify that the PVT receives glutamatergic projections from the PBN that promote aversive behavioral responses but do not modulate nociception. The latter finding is intriguing considering that the PBN is an important node in pain processing and that the PVT has recently emerged as a modulator of pain. Overall, the study includes an impressive array of techniques and manipulations and offers insight to an important scientific question. The authors' conclusions will be significantly strengthened by the inclusion of some additional experiments and controls.
It is in my view problematic that the authors used different genetic strategies to target the PBN-PVT pathway. For example, in Figure 1 the authors used Vglut2-cre mice for the anterograde tracings but later on in the same figure used constitutively expressed ChR2 in the PBN to assess functional connectivity with the PVT using ex-vivo patch-clamp electrophysiology. In Figure 2 the authors once again employed Vglut2-Cre mice to target PBN projections to the PVT and manipulate these projections optogenetically during behavioral tests. However, in the following figure (Fig. 3) the authors then use a retro-Cre approach and chemogenetics. The interchangeable use of these different manipulations is not warranted by data presented by the authors. For example it is unclear whether all PBN neurons projecting to the PVT are glutamatergic and express VGLUT2. When using the constitutively expensed ChR2 in the PBN to demonstrate glutamatergic projections to the PVT, the authors may be faced by potential contamination from adjacent brain stem structures like the LC and DRN, which project to the PVT and are known to contain glutamatergic neurons (vglut1 and vglut3, respectively). Another example, for figure 4 why did the authors not use Vglut2-cre mice and inhibited PBN terminals in the PVT as in Figure 2?
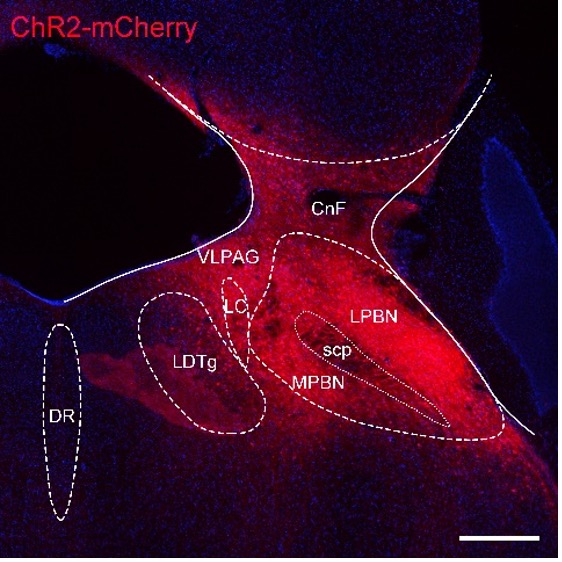
We agree with the reviewer. Now we have reframed this manuscript. We first presented the slice recording results from wild-type mice (Figure 1). We recorded both the EPSCs and IPSCs. We found that light-induced EPSCs in 34 of 52 neurons and light-induced IPSCs in 4 of 52 neurons. Please see Page 5 Line 119 to Line 121. We carefully examined the ChR2 virus infection area. Please see the following Fig R1 showcase. We found that there were dense ChR2-mCherry+ neurons in the PBN. We also observed ChR2-mCherry+ neurons in the nearby ventrolateral periaqueductal gray (VLPAG), locus coeruleus (LC), cuneiform nucleus (CnF), and laterodorsal tegmental nucleus (LDTg). And the dorsal raphe nucleus (DR) was not infected. We agreed with the reviewer that there could be potential contamination from the LC, which releases dopamine and norepinephrine to the PVT by LC-PVT projection. We have discussed this on Page 13 Line 375 to Line 380.
Figure R1. AAV-hSyn-ChR2-mCherry virus infection showcase. LPBN, lateral parabrachial nucleus. MPBN, medial parabrachial nucleus; VLPAG: ventrolateral periaqueductal gray; LC, locus coeruleus; CnF, cuneiform nucleus; LDTg, laterodorsal tegmental nucleus; DR, dorsal raphe nucleus; scp, superior cerebellar peduncle, scale bar: 200 μm.
We performed tdTomato staining with VgluT2 mRNA in situ hybridization and found that about 94.4% of tdTomato+ neurons express VgluT2 mRNA. These results indicate that the majority of PVT-projecting PBN neurons are glutamatergic. These new results have been included in Figure 1R−U.
Then we used VgluT2-ires-Cre mice to perform tracing (Figure1−figure supplement 2) and behavioral tests (optogenetic activation in Figure 2, optogenetic inhibition in Figure 4). We also performed the pharmacogenetic activation of PVT-projecting PBN neurons on wild-type mice (Figure 3). We observed that pharmacogenetic activation of the PVT-projecting PBN neurons reduced the center duration in the OFT, similar to the optogenetic activation OFT result. We also observed that pharmacogenetic activation of the PVT-projecting PBN neurons induced freezing behaviors. Our pharmacogenetic activation experiment supported the hypothesis that PBN-PVT projections modulate negative affective states.
Now we have now performed the optogenetic inhibition of the PBN-PVT projections using VgluT2-ires-Cre mice. We found that inhibition of PBN-PVT projections reduces 2-MT-induced aversion-like behaviors and footshock-induced freezing behaviors. These new results have been included in Figure 4, Figure 4−figure supplement 1 and 2, and were described in the text. Please see the text Page 9 Line 254 to Page 10 Line 274.
Related to the previous point, in the retrograde labeling experiment (Fig. 1) it would be useful if the authors determined what fraction of retrogradely label cells are indeed VGLUT2+. For behavioral experiments employing the retro-Cre approach the authors may be manipulating a heterogenous population of PBN neurons which could be influencing their behavioral observations. In general, the authors should ensure that a similar population of PBN-PVT neurons is been assessed throughout the study.
We have now performed tdTomato staining with VgluT2 mRNA in situ hybridization and found that approximately 94.4% of tdTomato+ neurons expressed VgluT2 mRNA. These results indicated that the majority of PVT-projecting PBN neurons are glutamatergic. These new results have been included in Figure 1R−U and were described in the text. Please see Page 5 Line 129 to Line 132.
The authors' grouping of the behavioral data into the first vs the last four minutes of light stimulation in the OF does not seem to be properly justified an appears rather arbitrary. Also related to data analysis, the unpaired t-test analysis in the fear conditioning experiment in Figure 4J seems inappropriate. ANOVA with group comparisons is more appropriate here.
To provide a more detailed profile of the behaviors in the OFT, we further divided the laser ON period (5−10 minutes) into five one-minute periods and analyzed the velocity, non-moving time, travel distance, center time, and jumping. We found that the velocity and non-moving time were increased, and the center time was decreased in the ChR2 mice during most periods. Furthermore, we observed that the travel distance and jumping behaviors were increased only in the first one-minute period in ChR2 mice. These new results have been included in Figure 2−figure supplement 2 and were described in the text. Please see Page 7 Line 179 to Line 189. We also discussed this on Page 14 Line 396 to Line 403.
We now performed the optogenetic inhibition of PBN-PVT projections in footshock-induced freezing behavior on Vglut2-ires-Cre mice (Figure 4J−K). And we revised the statistics (Unpaired student's t-test) and calculated the percentage of freezing behaviors in 10 minutes, which matched the constant optogenetic inhibition. Similar changes have been made in the Figure 4−figure supplement 3K.
Considering the persistency of the effect in the OF following optogenetic stimulation of PBN-PVT afferents, the lack of such persistent effect in the RTPA is hard to reconcile. By performing additional experiments the authors attempt to settle this discrepancy by proposing that the PBN-PVT pathway promotes aversion but does not facilitate negative associations. I find this conclusion to be problematic. If the pathway is critical for conveying aversive signals to the PVT, one expects that at the very least it would be require for the formation of associate memories involving aversive stimuli. However, the authors do not show data to this effect. Instead they show that animals decrease their acute defensive reactions to aversive stimuli (2-MT and fear conditioning), but do not show whether associative memory related to this experience (e.g. fear memory retrieval) is impacted by manipulations of the PBN-PVT pathway.
We have now performed several experiments to examine the effects of the PBN-PVT projections on aversion formation and memory retrieval.
We first performed a prolonged conditioned place aversion that mimics drug-induced place aversion. And we found that optogenetic activation of PBN-PVT projections did not induce aversion in the postconditioning test on Day 4. These new results have been included in Figure 2−figure supplement 2H−I and described in the text. Please see Page 7 Line 196 to Line 199.
Then, we performed the classical auditory fear conditioning test and found that optogenetic inhibition of PBN-PVT projections during footshock in the conditioning period did not affect freezing levels in contextual test or cue test (Laser OFF trials). And inhibition of PBN-PVT projections during contextual test or cue test (Laser On trials) did not affect freezing levels either. These data suggest that PBN-PVT projections are not crucial for associative fear memory formation or retrieval. These new results have been included in Figure 4−figure supplement 2 and described in the text. Please see Page 10 Line 268 to Page Line 274. We also discussed this on Page 15 Line 430 to Page 16 Line 473.
A similar lack of connection between aversive signals within the PVT and the PBN pathway is found in the photometry data presented in Figure 5. While importantly the authors' observation of aversive modulation of the pPVT reproduces data from other recent studies, the question here is whether the increased activity of PVT neurons is mediated by input from the PBN. The cFos experiment included in this figure attempts to draw this connection, but empirical evidence is required.
We have now performed the dual Fos staining experiment and the optoeletrode experiment.
In the dual Fos staining experiment, we found that there was a broad overlap between optogenetic stimulation-activated neurons (expressing the Fos protein) and footshock-activated neurons (expressing the fos mRNA) (Figure 6−figure supplement 1B−E).
In optoelectrode experiment, there was also a broad overlap between laser-activated and footshock-activated neurons. This result was consistent with the dual Fos staining result, suggesting that PVTPBN neurons were activated by aversive stimulation. Next, we analyzed the firing rates of PVT neurons during footshock with laser sweeps and footshock without laser sweeps. We found that the footshock stimulus with laser activated 30 of 40 neurons and increased the overall firing rates of 40 neurons compared with the footshock without laser result (Figure 6I). These results indicated that activation of PBN-PVT projections could enhance PVT neuronal responses to aversive stimulation.
These new results have been included in Figure 6, Figure 6−figure supplement 1, and described in the text. Please see Page 10 Line 295 to Page 11 Line 317. We also discussed these results on Page 15 Line 422 to Line 429.
Reviewer #2 (Public Review):
Zhu et al. investigated the connectivity and functional role of the projections from the parabrachial nucleus (PBN) to the paraventricular nucleus of the thalamus (PVT). Using neural tracers and in vitro electrophysiological recordings, the authors showed the existence of monosynaptic glutamatergic connections between the PBN and PVT. Further behavioral tests using optogenetic and chemogenetic approaches demonstrated that activation of the PVT-PBN circuit induces aversive and anxiety-like behaviors, whereas optogenetic inhibition of PVT-projecting PBN neurons reduces fear and aversive responses elicited by footshock or the synthetic predator odor 2MT. Next, they characterized the anatomical targets of PVT neurons that receive direct innervation from the PBN (PVTPBN). The authors also showed that PVTPBN neurons are activated by aversive stimuli and chemogenetically exciting these cells is sufficient to induce anxiety-like behaviors. While the data mostly support their conclusions, alternative interpretations and potential caveats should be addressed in the discussion.
Strength:
The authors used different behavioral tests that collectively support a role for PBN-PVT projections in promoting fear- and anxiety-like behaviors, but not nociceptive or depressive-like responses. They also provided insights into the temporal participation of the PBN-PVT circuit by showing that this pathway regulates the expression of affective states without contributing for the formation of fear-associated memories. Because previous studies have shown that activation of projection-defined PVT neurons is sufficient to induce the formation of aversive memories, the differences between the present study and previous findings reinforce the idea of functional heterogeneity within the PVT. The authors further explored this functional heterogeneity in PVT by using an anterograde viral construct to selectively label PVT neurons that are targeted by PBN inputs. Together, these results connect two important brain regions (i.e., PBN and PVT) that were known to be involved in fear and aversive responses, and provide new information to help the field to elucidate the complex networks that control emotional behaviors.
Weakness:
The authors should avoid anthropomorphizing the behavioral interpretation of the findings and generalizing their conclusions. In addition, there is a series of potential caveats that could interfere with the interpretation of the results, all of which must be discussed in the article. For example, the long protocol duration of laser stimulation, the possibility of antidromic effects following photoactivation of PBN terminals in PVT, and the existence of collateral PBN projections that could also be contributing for the observed behavioral changes. Additional clarification about the exclusive glutamatergic nature of the PBN-PVT projection should be provided and the present findings should be reconciled with prior studies showing the existence of GABAergic PBN-PVT projections.
We agree with the reviewer. Now we have revised the text carefully to avoid using subjective terms. We showed the light-induced EPSCs and IPSCs results in Figure 1, and we performed RNAscope experiments to clarify the glutamatergic nature of the PVT-projecting PBN neurons (Figure 1 and Figure1−figure supplement 1). We also added discussion about the laser stimulation protocol, the potential possibility of antidromic effects, and collateral projections. Please see Page 14 Line 413 to Page 15 Line 418, and Page 16 Line 449 to Line 457.
We also added several experiments to dissect the effect of manipulation of the PBN-PVT projection in fear memory acquisition and retrieval. These new results have been included in Figure 4−figure supplement 2 and described in the text. Please see Page 10 Line 268 to Line 274. We also discussed this on Page 15 Line 430 to Page 16 Line 473.
Reviewer #3 (Public Review):
Zhu YB et al investigated the functional role of the parabrachial nucleus (PBN) to the thalamic paraventricular nucleus (PVT) in processing negative emotions. They found that PBN send excitatory projection to PVT. The activation of PBN-PVT projection induces anxiety-like and fear-like behaviors, while inhibition of this projection relieves fear and aversion.
Strengths:
The authors dissected anatomic and functional connection between the PBN and the PVT by using comprehensive modern neuroscience techniques including viral tracing, electrophysiology, optogenetics and pharmacogenetics. They clearly demonstrated the significant role of PBN-PVT projection in modulating negative emotions.
Weaknesses:
The PBN contains a variety of neuronal subtypes that expressed distinct molecular marker such as CGRP, Tac1, Pdyn, Nts et al. The PBN also send projections to multiple targets, including VMH, PAG, BNST, CEA and ILN that could mediate distinct function. What's the neuronal identity of PVT-projecting PBN neurons, how is the PVT projection and other projections organized, are they overlapping or relative independent pathway? Those important questions were not examined in this study, which make it hard to relate this finding to other existing literature.
We have now performed the RNAscope experiments detecting VgluT2, Tac1, Tacr1, Pdyn mRNA, and fluorescent immunostaining detecting CGRP protein in the PBN. We found that about 94.4% of tdTomato+ neurons express VgluT2 mRNA. We also found that tdTomato+ neurons were only partially co-labeled with Tacr1, Tac1, or Pdyn mRNA, but not with CGRP. These results indicate that the majority of PVT-projecting PBN neurons are glutamatergic. These new results have been included in Figure 1, Figure 1−figure supplement 1, and were described in the text. Please see Page 5 Line 129 to Line 140.
We also provided the collateral projections from PVT-projecting neurons in Figure 1−figure supplement 3, Page 6 Line 148 to Line 151, and discussed on Page 16 Line 449 to Line 457.
-

Evaluation Summary:
This study will interest neuroscientists, in particular those interested in the neurocircuitry of emotional behaviors. Using modern neuroscience techniques, the authors demonstrate that anatomical projections from a brain stem structure called the parabrachial nucleus to the paraventricular nucleus thalamus contribute to aversive states like fear and anxiety. Overall, the study offers important details of a previously uncharacterized brain circuit, although some additional experiments are required to fully substantiate the authors' claims.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
In their manuscript entitled "PBN-PVT projection modulates negative emotions in mice", Zhu et al. combine circuit mapping techniques with behavioral manipulations to interrogate the function of anatomical projections from the parabrachial nucleus (PBN) to the paraventricular nucleus of the thalamus (PVT). The study addresses an important scientific question, since the PVT and particularly the posterior PVT is known to be mostly sensitive to aversive signals, but the neural circuit mechanisms underlying this process remain unknown. Here the authors contribute important evidence that PBN inputs to the PVT may be critical for this process. Specifically, the authors identify that the PVT receives glutamatergic projections from the PBN that promote aversive behavioral responses but do not modulate nociception. …
Reviewer #1 (Public Review):
In their manuscript entitled "PBN-PVT projection modulates negative emotions in mice", Zhu et al. combine circuit mapping techniques with behavioral manipulations to interrogate the function of anatomical projections from the parabrachial nucleus (PBN) to the paraventricular nucleus of the thalamus (PVT). The study addresses an important scientific question, since the PVT and particularly the posterior PVT is known to be mostly sensitive to aversive signals, but the neural circuit mechanisms underlying this process remain unknown. Here the authors contribute important evidence that PBN inputs to the PVT may be critical for this process. Specifically, the authors identify that the PVT receives glutamatergic projections from the PBN that promote aversive behavioral responses but do not modulate nociception. The latter finding is intriguing considering that the PBN is an important node in pain processing and that the PVT has recently emerged as a modulator of pain. Overall, the study includes an impressive array of techniques and manipulations and offers insight to an important scientific question. The authors' conclusions will be significantly strengthened by the inclusion of some additional experiments and controls.
It is in my view problematic that the authors used different genetic strategies to target the PBN-PVT pathway. For example, in Figure 1 the authors used Vglut2-cre mice for the anterograde tracings but later on in the same figure used constitutively expressed ChR2 in the PBN to assess functional connectivity with the PVT using ex-vivo patch-clamp electrophysiology. In Figure 2 the authors once again employed Vglut2-Cre mice to target PBN projections to the PVT and manipulate these projections optogenetically during behavioral tests. However, in the following figure (Fig. 3) the authors then use a retro-Cre approach and chemogenetics. The interchangeable use of these different manipulations is not warranted by data presented by the authors. For example it is unclear whether all PBN neurons projecting to the PVT are glutamatergic and express VGLUT2. When using the constitutively expensed ChR2 in the PBN to demonstrate glutamatergic projections to the PVT, the authors may be faced by potential contamination from adjacent brain stem structures like the LC and DRN, which project to the PVT and are known to contain glutamatergic neurons (vglut1 and vglut3, respectively). Another example, for figure 4 why did the authors not use Vglut2-cre mice and inhibited PBN terminals in the PVT as in Figure 2?
Related to the previous point, in the retrograde labeling experiment (Fig. 1) it would be useful if the authors determined what fraction of retrogradely label cells are indeed VGLUT2+. For behavioral experiments employing the retro-Cre approach the authors may be manipulating a heterogenous population of PBN neurons which could be influencing their behavioral observations. In general, the authors should ensure that a similar population of PBN-PVT neurons is been assessed throughout the study.
The authors' grouping of the behavioral data into the first vs the last four minutes of light stimulation in the OF does not seem to be properly justified an appears rather arbitrary. Also related to data analysis, the unpaired t-test analysis in the fear conditioning experiment in Figure 4J seems inappropriate. ANOVA with group comparisons is more appropriate here.
Considering the persistency of the effect in the OF following optogenetic stimulation of PBN-PVT afferents, the lack of such persistent effect in the RTPA is hard to reconcile. By performing additional experiments the authors attempt to settle this discrepancy by proposing that the PBN-PVT pathway promotes aversion but does not facilitate negative associations. I find this conclusion to be problematic. If the pathway is critical for conveying aversive signals to the PVT, one expects that at the very least it would be require for the formation of associate memories involving aversive stimuli. However, the authors do not show data to this effect. Instead they show that animals decrease their acute defensive reactions to aversive stimuli (2-MT and fear conditioning), but do not show whether associative memory related to this experience (e.g. fear memory retrieval) is impacted by manipulations of the PBN-PVT pathway.
A similar lack of connection between aversive signals within the PVT and the PBN pathway is found in the photometry data presented in Figure 5. While importantly the authors' observation of aversive modulation of the pPVT reproduces data from other recent studies, the question here is whether the increased activity of PVT neurons is mediated by input from the PBN. The cFos experiment included in this figure attempts to draw this connection, but empirical evidence is required.
-

Reviewer #2 (Public Review):
Zhu et al. investigated the connectivity and functional role of the projections from the parabrachial nucleus (PBN) to the paraventricular nucleus of the thalamus (PVT). Using neural tracers and in vitro electrophysiological recordings, the authors showed the existence of monosynaptic glutamatergic connections between the PBN and PVT. Further behavioral tests using optogenetic and chemogenetic approaches demonstrated that activation of the PVT-PBN circuit induces aversive and anxiety-like behaviors, whereas optogenetic inhibition of PVT-projecting PBN neurons reduces fear and aversive responses elicited by footshock or the synthetic predator odor 2MT. Next, they characterized the anatomical targets of PVT neurons that receive direct innervation from the PBN (PVTPBN). The authors also showed that PVTPBN …
Reviewer #2 (Public Review):
Zhu et al. investigated the connectivity and functional role of the projections from the parabrachial nucleus (PBN) to the paraventricular nucleus of the thalamus (PVT). Using neural tracers and in vitro electrophysiological recordings, the authors showed the existence of monosynaptic glutamatergic connections between the PBN and PVT. Further behavioral tests using optogenetic and chemogenetic approaches demonstrated that activation of the PVT-PBN circuit induces aversive and anxiety-like behaviors, whereas optogenetic inhibition of PVT-projecting PBN neurons reduces fear and aversive responses elicited by footshock or the synthetic predator odor 2MT. Next, they characterized the anatomical targets of PVT neurons that receive direct innervation from the PBN (PVTPBN). The authors also showed that PVTPBN neurons are activated by aversive stimuli and chemogenetically exciting these cells is sufficient to induce anxiety-like behaviors. While the data mostly support their conclusions, alternative interpretations and potential caveats should be addressed in the discussion.
Strength:
The authors used different behavioral tests that collectively support a role for PBN-PVT projections in promoting fear- and anxiety-like behaviors, but not nociceptive or depressive-like responses. They also provided insights into the temporal participation of the PBN-PVT circuit by showing that this pathway regulates the expression of affective states without contributing for the formation of fear-associated memories. Because previous studies have shown that activation of projection-defined PVT neurons is sufficient to induce the formation of aversive memories, the differences between the present study and previous findings reinforce the idea of functional heterogeneity within the PVT. The authors further explored this functional heterogeneity in PVT by using an anterograde viral construct to selectively label PVT neurons that are targeted by PBN inputs. Together, these results connect two important brain regions (i.e., PBN and PVT) that were known to be involved in fear and aversive responses, and provide new information to help the field to elucidate the complex networks that control emotional behaviors.
Weakness:
The authors should avoid anthropomorphizing the behavioral interpretation of the findings and generalizing their conclusions. In addition, there is a series of potential caveats that could interfere with the interpretation of the results, all of which must be discussed in the article. For example, the long protocol duration of laser stimulation, the possibility of antidromic effects following photoactivation of PBN terminals in PVT, and the existence of collateral PBN projections that could also be contributing for the observed behavioral changes. Additional clarification about the exclusive glutamatergic nature of the PBN-PVT projection should be provided and the present findings should be reconciled with prior studies showing the existence of GABAergic PBN-PVT projections.
-

Reviewer #3 (Public Review):
Zhu YB et al investigated the functional role of the parabrachial nucleus (PBN) to the thalamic paraventricular nucleus (PVT) in processing negative emotions. They found that PBN send excitatory projection to PVT. The activation of PBN-PVT projection induces anxiety-like and fear-like behaviors, while inhibition of this projection relieves fear and aversion.
Strengths:
The authors dissected anatomic and functional connection between the PBN and the PVT by using comprehensive modern neuroscience techniques including viral tracing, electrophysiology, optogenetics and pharmacogenetics. They clearly demonstrated the significant role of PBN-PVT projection in modulating negative emotions.
Weaknesses:
The PBN contains a variety of neuronal subtypes that expressed distinct molecular marker such as CGRP, Tac1, Pdyn, …
Reviewer #3 (Public Review):
Zhu YB et al investigated the functional role of the parabrachial nucleus (PBN) to the thalamic paraventricular nucleus (PVT) in processing negative emotions. They found that PBN send excitatory projection to PVT. The activation of PBN-PVT projection induces anxiety-like and fear-like behaviors, while inhibition of this projection relieves fear and aversion.
Strengths:
The authors dissected anatomic and functional connection between the PBN and the PVT by using comprehensive modern neuroscience techniques including viral tracing, electrophysiology, optogenetics and pharmacogenetics. They clearly demonstrated the significant role of PBN-PVT projection in modulating negative emotions.
Weaknesses:
The PBN contains a variety of neuronal subtypes that expressed distinct molecular marker such as CGRP, Tac1, Pdyn, Nts et al. The PBN also send projections to multiple targets, including VMH, PAG, BNST, CEA and ILN that could mediate distinct function. What's the neuronal identity of PVT-projecting PBN neurons, how is the PVT projection and other projections organized, are they overlapping or relative independent pathway? Those important questions were not examined in this study, which make it hard to relate this finding to other existing literature.
-