Two different cell-cycle processes determine the timing of cell division in Escherichia coli
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
How the bacterium E.coli decides when to divide is an interesting, important, unsolved and highly controversial topic of interest to readers across disciplines, including microbiology, cell biology and statistical physics. Popular "single process" models invoke regulation at the step of replication initiation or at the step of cell division per se, whereas these authors have previously proposed a "concurrent cycles" model in which both processes are relevant, with different prominences in different situations. Consistent with the authors' motivating hypothesis, in the particular perturbed condition investigated in this work, a process different from DNA replication becomes increasingly important for division control as the degree of perturbation increases, which provides a new challenge to models for cell division control.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 and Reviewer #3 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Cells must control the cell cycle to ensure that key processes are brought to completion. In Escherichia coli , it is controversial whether cell division is tied to chromosome replication or to a replication-independent inter-division process. A recent model suggests instead that both processes may limit cell division with comparable odds in single cells. Here, we tested this possibility experimentally by monitoring single-cell division and replication over multiple generations at slow growth. We then perturbed cell width, causing an increase of the time between replication termination and division. As a consequence, replication became decreasingly limiting for cell division, while correlations between birth and division and between subsequent replication-initiation events were maintained. Our experiments support the hypothesis that both chromosome replication and a replication-independent inter-division process can limit cell division: the two processes have balanced contributions in non-perturbed cells, while our width perturbations increase the odds of the replication-independent process being limiting.
Article activity feed
-

-

Author Response:
Reviewer #1:
The manuscript "Two different cell-cycle processes determine the timing of cell division in Escherichia coli" by Colin et al. presents an experimental approach to investigate the role of two governing cell-cycle processes, namely, DNA replication-segregation and cell division cycle, in size regulation. Authors tackle the problem by first decoupling these two cell-cycle process via sub-lethal dosages of A22, and then analyze the role of each process in the timing of cell division. Modern imaging and analysis techniques are used in this work to monitor cell division with single-cell resolution and chromosome replication with sub-cellular resolution. The large pool of data allows the authors to perform correlation analysis of cell-size and the cell cycle parameters, which led to the conclusion that the two …
Author Response:
Reviewer #1:
The manuscript "Two different cell-cycle processes determine the timing of cell division in Escherichia coli" by Colin et al. presents an experimental approach to investigate the role of two governing cell-cycle processes, namely, DNA replication-segregation and cell division cycle, in size regulation. Authors tackle the problem by first decoupling these two cell-cycle process via sub-lethal dosages of A22, and then analyze the role of each process in the timing of cell division. Modern imaging and analysis techniques are used in this work to monitor cell division with single-cell resolution and chromosome replication with sub-cellular resolution. The large pool of data allows the authors to perform correlation analysis of cell-size and the cell cycle parameters, which led to the conclusion that the two processes have a "balanced contributions in non-perturbed cells."
The question studied in this manuscript is important and timely. The investigation of the two concurrent processes chosen by the authors is perhaps the right direction which may eventually lead to a complete understanding of the E. coli cell-cycle and size regulation. The high-resolution imaging and analysis accomplished in this work is also commendable. There is, however, a major concern about this manuscript, which is the entire conclusion is based on the cell-cycle and size perturbations by A22. The caveat of the A22 perturbations is that an aberrant cell shape could affect both of the cellular processes simultaneously. Even though the C-period and initiation size are largely unchanged, a possible, but unknown, cross-talk between the two processes may be affected by A22. Therefore, additional evidence is necessary to show whether the two processes independently determine cell division.
We agree that A22 treatment could possibly affect DNA replication or organization, e.g., indirectly through an effect of cell width on DNA organization. It would thus indeed be desirable to confirm our findings based on alternative perturbations. At the same time, our experiments clearly demonstrate that cell sizes at replication initiation and division are decreasingly correlated with increasing A22 concentration, which suggests that a process different from DNA replication is responsible for the timing of division.
Additionally, DNA replication could depend on cell division, which could possibly complicate the relationship between replication and division. We have now addressed the possibility of an influence on division on replication initiation in the Discussion, where we write ‘The concurrent-cycles framework assumes that replication initiation is independent of cell division or cell size at birth, [...]. However, we note that this is not the only possibility, and DNA replication may not be entirely independent of cell division. A complementary hypothesis \citep{Kleckner2018} posits a possible (additional or complementary) connection of initiation to the preceding division event. To test this hypothesis one could perturb specific division processes by titrating components involved in Z-ring assembly (e.g., titrating FtsZ \citep{Zheng2016}).’
Reviewer #2:
This is an interesting paper which makes important contributions to an interesting and highly controversial topic: how does an E.coli cell decide when to divide.
As the authors describe in clear and careful detail, two main camps have argued (often dogmatically) for "single process" models in which division is either a direct, downstream consequence of replication initiation (which is the regulated step) or of effects that act directly on division (irrespective of replication and, more generally, the chromosome cycle). The authors of this paper have, instead, proposed that both types of effects are important, in different proportions according to the circumstances. They refer to this idea as a "concurrent cycles" hypothesis. In previous work they have presented arguments and data which they interpret as being incompatible with any single process model and consistent with their alternative hypothesis.
This work now investigates the consequences of treatment with A22, a drug which inhibits MreB, with the result that it increases cell width and, concomitantly, increases the length of time between completion of a given round of DNA replication and the immediately ensuing cell division (an interval known as the "D period"). The idea to analyze this situation was motivated by the authors previous hypothesis: by the concurrent cycles idea, increasing the length of the D-period should prolong the replication-independent inter-division process such that it becomes rate limiting in determining the timing of division (relative to the replication-dependent process).
The data presented confirm the authors' expectation. They first show that progressively increasing the amount of A22 does not (dramatically) alter either: (i) the basic "adder" behavior in which a fixed amount of cell length is added irrespective of the length of the cell at birth or (ii) the finding that a fixed amount of cell length is added per replication origin during the period from one round of replication initiation to the next, which is consistent with (and generally considered to be supportive of) a role for a replication-dependent process.
However, they also discover an interesting additional effect by examining the amount of cell length added (per origin) during the entire period comprising replication plus the immediately ensuing division ("C+D"). In the unperturbed case, cells that are longer at the time of initiation of replication also add more length during the ensuing (C+D) period. In contrast, in the presence of increasing amounts of A22, this effect is progressively reversed such that, finally, at high drug levels, cells which are longer (per origin) at the time of initiation of replication add much less length during the ensuing (C+D) period. Since the length of the C period is essentially constant in all conditions, the relevant effect is the variation in the length of the D period. And since the observed effect becomes more and more prominent with increasing A22 concentration, variation in the D period dominates more and more as the length of that period gets longer and longer. The authors interpret this effect to mean that, with increasing D-period length, division timing is decreasingly dependent on replication initiation. They go on to infer that "with increasing average D period, a process different from DNA replication is likely increasingly responsible for division control". This is a sensible, relatively formal restatement of the finding. This statement allows for diverse specific interpretations. The authors focus on one possible interpretation: they show that their previously proposed concurrent cycles hypothesis can quantitatively explain these data. In essence, given a replication-independent and a replication-dependent process, the observed findings are explained by an increased contribution of the replication-independent process. This scenario also does a better job of explaining the presented data, as well as other findings, than other recent "single process" models, for reasons that are discussed in straightforward detail in the Discussion. The authors also do an excellent job of laying out the assumptions upon which their model (and other existing models) are based, thus laying open the possibility for future studies to consider other possible scenarios.
This work is important for four reasons. First, provides interesting new data which must be accommodated by any synthetic explanation for cell division control. Second, it makes it abundantly clear that the validity of any proposed single process model remains to be further substantiated. Third, it suggests an interesting alternative model which can accommodate a diversity of data, including that presented in the current work, and which has the potentially attractive feature of combining the two existing single-process models. Fourth, and perhaps most importantly, the authors discussion of the available data in this field clear, thoughtful and thought-provoking and leaves open the possibility of some as-yet unimagined mechanism. Overall, this work provides an important counterpoint to other published work and is a very valuable contribution to thinking and discussion in this field.
[It can also be noted specifically that this work provides an important counterpoint to the model proposed in a previous eLIFE paper on this topic by Witz et al., 2019 (eLife 2019;8:e48063 doi: 10.7554/eLife.48063).]
We thank the reviewer for her careful assessment and appreciation of our work.
Reviewer #3:
Colin, Micali et al. investigated slow-growing E. coli cells' division and replication over cell cycles at single cell level with the perturbed cellular dimension. They found that the time between replication termination and division increased by perturbing cell width as recently reported, and that chromosome replication became decreasingly limiting for cell division. These results well supported the 'concurrent-processes model' previously proposed by some of the authors.
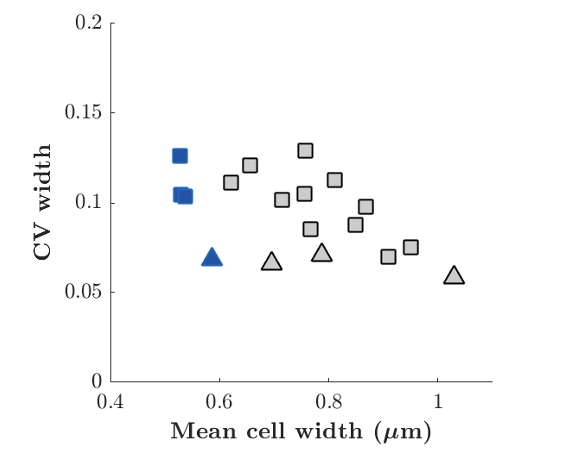
- Cell length can be used to represent the cell size (adder) only if the cell width keeps constant. In the current form of the manuscript, it is unknown whether or not the cell width varies significantly at single-cell level with A22 treatment (e.g., 1µg/ml A22). In this case, cell volume might not be nicely correlated with cell length. The interpretation of Figure 3 therefore would be devalued.
We now demonstrate in the new Figure 2–S2 that the coefficient of variations of cell width does not increase with A22 concentration (neither in snapshots from cells grown in liquid culture nore in the mother machine):
Figure: Variation of width at the single-cell level. Coefficient of variation of cell width as a function of mean cell with. Squares and triangles represent measurements done on cells grown in mother machine or in liquid culture respectively. Blue color represents wild-type cells. Grey color represents cells treated with different amounts of A22.
We also reference this figure in the main text, writing: ‘Increasing A22 concentration leads to increasing steady-state cell width both in batch culture and in the mother machine (Figure \ref{fig2}B), without affecting cell-to-cell width fluctuations (Figure \ref{CV_width}),} and without affecting doubling time (Figure \ref{fig2}C) or single-cell growth rate (Figure \ref{SI_Fig1}).’
- The negative value of 𝜁C+D in Figure 3F (treated group) indicates that the division length is negatively correlated with the cell length at replication initiation. It is not obvious that this can rule out the possible contribution of DNA replication/segregation in offsetting the length difference at initiation and thus contribute to cell division. Since Figure 3F is the key observation to validate the model, more explanations are required to help readers understand how a negative 𝜁C+D can lead to a conclusion that a process different from DNA replication is likely responsible for division control with A22-treatment.
The negative value of zeta_CD actually corresponds to a lack of correlation between division size and size at initiation, typically predicted by the models where replication is never limiting for cell division (Micali et al 2018, Si et al. 2019). We have commented more explicitly on this point in the text, writing: ‘Note that the negative value of $zeta_{\rm CD}$ corresponds to a lack of correlation between division size and size at initiation (Figure \ref{fig3}G), typically predicted by the models where replication is never limiting for cell division~\cite{Micali2018,Micali2018b,Si2019}.’
- As an important input for the model, the QC+D' is assumed to be equal to QC+D in unperturbed conditions and remains constant regardless of the A22 concentration (Line 548-554). This assumption is reasonable if the minimum time interval for segregation (D') is irrelevant to the change of cell width. But how D' and QC+D' changes with cell width are unknown. Earlier molecular studies revealed that the polymerization of MreB affects the activity of topoisomerase IV, an enzyme mediates the dimerization of sister chromosomes, which implies that changing cell width may affect D'. Given the importance of QC+D' to the model, it is vital for the authors to make this assumption clear in maintext and explain why such assumption is reasonable.
Q_CD’ (related to average growth in the CD’ period) is a parameter that we cannot measure, or bypass in the model. We have made this assumption more explicit in the text. While this question deserves further investigation in future studies, we know that D’ cannot increase too strongly with width, because otherwise it would leave replication/segregation limiting for division under A22 perturbations, contrary to our observation. This is the main reason to assume D’ constant in the model. A posteriori we can say that the loss of correlation between size at division and size at initiation observed under A22 treatment is in line with the hypothesis that D’ does not increase too much in order for the segregation process to interfere with cell division. We now write: ‘Note that neither the minimum completion time C+D' nor the coupling parameter $zeta_{CD’}$ can be measured experimentally, or bypassed in the model. In principle these parameters could change under A22 perturbations, since MreB affects the activity of topoisomerase IV \citep{madabhushi2009actin,kruse2003dysfunctional}, an enzyme that mediates the dimerization of sister chromosomes. However, constancy of $\zeta_{CD'}$ is supported by the constancy of the C period, and the minimum D' period cannot increase too strongly with width in the model, because otherwise it would render replication/segregation limiting for division under A22 perturbations, contrary to our experimental observation. Hence, for simplicity, we assumed $\zeta_{CD'}$ and the D' period to stay constant.’
-

Evaluation Summary:
How the bacterium E.coli decides when to divide is an interesting, important, unsolved and highly controversial topic of interest to readers across disciplines, including microbiology, cell biology and statistical physics. Popular "single process" models invoke regulation at the step of replication initiation or at the step of cell division per se, whereas these authors have previously proposed a "concurrent cycles" model in which both processes are relevant, with different prominences in different situations. Consistent with the authors' motivating hypothesis, in the particular perturbed condition investigated in this work, a process different from DNA replication becomes increasingly important for division control as the degree of perturbation increases, which provides a new challenge to models for cell division …
Evaluation Summary:
How the bacterium E.coli decides when to divide is an interesting, important, unsolved and highly controversial topic of interest to readers across disciplines, including microbiology, cell biology and statistical physics. Popular "single process" models invoke regulation at the step of replication initiation or at the step of cell division per se, whereas these authors have previously proposed a "concurrent cycles" model in which both processes are relevant, with different prominences in different situations. Consistent with the authors' motivating hypothesis, in the particular perturbed condition investigated in this work, a process different from DNA replication becomes increasingly important for division control as the degree of perturbation increases, which provides a new challenge to models for cell division control.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 and Reviewer #3 agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
The manuscript "Two different cell-cycle processes determine the timing of cell division in Escherichia coli" by Colin et al. presents an experimental approach to investigate the role of two governing cell-cycle processes, namely, DNA replication-segregation and cell division cycle, in size regulation. Authors tackle the problem by first decoupling these two cell-cycle process via sub-lethal dosages of A22, and then analyze the role of each process in the timing of cell division. Modern imaging and analysis techniques are used in this work to monitor cell division with single-cell resolution and chromosome replication with sub-cellular resolution. The large pool of data allows the authors to perform correlation analysis of cell-size and the cell cycle parameters, which led to the conclusion that the two …
Reviewer #1 (Public Review):
The manuscript "Two different cell-cycle processes determine the timing of cell division in Escherichia coli" by Colin et al. presents an experimental approach to investigate the role of two governing cell-cycle processes, namely, DNA replication-segregation and cell division cycle, in size regulation. Authors tackle the problem by first decoupling these two cell-cycle process via sub-lethal dosages of A22, and then analyze the role of each process in the timing of cell division. Modern imaging and analysis techniques are used in this work to monitor cell division with single-cell resolution and chromosome replication with sub-cellular resolution. The large pool of data allows the authors to perform correlation analysis of cell-size and the cell cycle parameters, which led to the conclusion that the two processes have a "balanced contributions in non-perturbed cells."
The question studied in this manuscript is important and timely. The investigation of the two concurrent processes chosen by the authors is perhaps the right direction which may eventually lead to a complete understanding of the E. coli cell-cycle and size regulation. The high-resolution imaging and analysis accomplished in this work is also commendable. There is, however, a major concern about this manuscript, which is the entire conclusion is based on the cell-cycle and size perturbations by A22. The caveat of the A22 perturbations is that an aberrant cell shape could affect both of the cellular processes simultaneously. Even though the C-period and initiation size are largely unchanged, a possible, but unknown, cross-talk between the two processes may be affected by A22. Therefore, additional evidence is necessary to show whether the two processes independently determine cell division.
-

Reviewer #2 (Public Review):
This is an interesting paper which makes important contributions to an interesting and highly controversial topic: how does an E.coli cell decide when to divide.
As the authors describe in clear and careful detail, two main camps have argued (often dogmatically) for "single process" models in which division is either a direct, downstream consequence of replication initiation (which is the regulated step) or of effects that act directly on division (irrespective of replication and, more generally, the chromosome cycle). The authors of this paper have, instead, proposed that both types of effects are important, in different proportions according to the circumstances. They refer to this idea as a "concurrent cycles" hypothesis. In previous work they have presented arguments and data which they interpret as …
Reviewer #2 (Public Review):
This is an interesting paper which makes important contributions to an interesting and highly controversial topic: how does an E.coli cell decide when to divide.
As the authors describe in clear and careful detail, two main camps have argued (often dogmatically) for "single process" models in which division is either a direct, downstream consequence of replication initiation (which is the regulated step) or of effects that act directly on division (irrespective of replication and, more generally, the chromosome cycle). The authors of this paper have, instead, proposed that both types of effects are important, in different proportions according to the circumstances. They refer to this idea as a "concurrent cycles" hypothesis. In previous work they have presented arguments and data which they interpret as being incompatible with any single process model and consistent with their alternative hypothesis.
This work now investigates the consequences of treatment with A22, a drug which inhibits MreB, with the result that it increases cell width and, concomitantly, increases the length of time between completion of a given round of DNA replication and the immediately ensuing cell division (an interval known as the "D period"). The idea to analyze this situation was motivated by the authors previous hypothesis: by the concurrent cycles idea, increasing the length of the D-period should prolong the replication-independent inter-division process such that it becomes rate limiting in determining the timing of division (relative to the replication-dependent process).
The data presented confirm the authors' expectation.
They first show that progressively increasing the amount of A22 does not (dramatically) alter either: (i) the basic "adder" behavior in which a fixed amount of cell length is added irrespective of the length of the cell at birth or (ii) the finding that a fixed amount of cell length is added per replication origin during the period from one round of replication initiation to the next, which is consistent with (and generally considered to be supportive of) a role for a replication-dependent process.
However, they also discover an interesting additional effect by examining the amount of cell length added (per origin) during the entire period comprising replication plus the immediately ensuing division ("C+D"). In the unperturbed case, cells that are longer at the time of initiation of replication also add more length during the ensuing (C+D) period. In contrast, in the presence of increasing amounts of A22, this effect is progressively reversed such that, finally, at high drug levels, cells which are longer (per origin) at the time of initiation of replication add much less length during the ensuing (C+D) period. Since the length of the C period is essentially constant in all conditions, the relevant effect is the variation in the length of the D period. And since the observed effect becomes more and more prominent with increasing A22 concentration, variation in the D period dominates more and more as the length of that period gets longer and longer. The authors interpret this effect to mean that, with increasing D-period length, division timing is decreasingly dependent on replication initiation. They go on to infer that "with increasing average D period, a process different from DNA replication is likely increasingly responsible for division control". This is a sensible, relatively formal restatement of the finding. This statement allows for diverse specific interpretations. The authors focus on one possible interpretation: they show that their previously proposed concurrent cycles hypothesis can quantitatively explain these data. In essence, given a replication-independent and a replication-dependent process, the observed findings are explained by an increased contribution of the replication-independent process. This scenario also does a better job of explaining the presented data, as well as other findings, than other recent "single process" models, for reasons that are discussed in straightforward detail in the Discussion. The authors also do an excellent job of laying out the assumptions upon which their model (and other existing models) are based, thus laying open the possibility for future studies to consider other possible scenarios.This work is important for four reasons. First, provides interesting new data which must be accommodated by any synthetic explanation for cell division control. Second, it makes it abundantly clear that the validity of any proposed single process model remains to be further substantiated. Third, it suggests an interesting alternative model which can accommodate a diversity of data, including that presented in the current work, and which has the potentially attractive feature of combining the two existing single-process models. Fourth, and perhaps most importantly, the authors discussion of the available data in this field clear, thoughtful and thought-provoking and leaves open the possibility of some as-yet unimagined mechanism. Overall, this work provides an important counterpoint to other published work and is a very valuable contribution to thinking and discussion in this field.
[It can also be noted specifically that this work provides an important counterpoint to the model proposed in a previous eLIFE paper on this topic by Witz et al., 2019 (eLife 2019;8:e48063 doi: 10.7554/eLife.48063).]
-

Reviewer #3 (Public Review):
Colin, Micali et al. investigated slow-growing E. coli cells' division and replication over cell cycles at single cell level with the perturbed cellular dimension. They found that the time between replication termination and division increased by perturbing cell width as recently reported, and that chromosome replication became decreasingly limiting for cell division. These results well supported the 'concurrent-processes model' previously proposed by some of the authors.
Cell length can be used to represent the cell size (adder) only if the cell width keeps constant. In the current form of the manuscript, it is unknown whether or not the cell width varies significantly at single-cell level with A22 treatment (e.g., 1µg/ml A22). In this case, cell volume might not be nicely correlated with cell length. The …
Reviewer #3 (Public Review):
Colin, Micali et al. investigated slow-growing E. coli cells' division and replication over cell cycles at single cell level with the perturbed cellular dimension. They found that the time between replication termination and division increased by perturbing cell width as recently reported, and that chromosome replication became decreasingly limiting for cell division. These results well supported the 'concurrent-processes model' previously proposed by some of the authors.
Cell length can be used to represent the cell size (adder) only if the cell width keeps constant. In the current form of the manuscript, it is unknown whether or not the cell width varies significantly at single-cell level with A22 treatment (e.g., 1µg/ml A22). In this case, cell volume might not be nicely correlated with cell length. The interpretation of Figure 3 therefore would be devalued.
The negative value of 𝜁C+D in Figure 3F (treated group) indicates that the division length is negatively correlated with the cell length at replication initiation. It is not obvious that this can rule out the possible contribution of DNA replication/segregation in offsetting the length difference at initiation and thus contribute to cell division. Since Figure 3F is the key observation to validate the model, more explanations are required to help readers understand how a negative 𝜁C+D can lead to a conclusion that a process different from DNA replication is likely responsible for division control with A22-treatment.
As an important input for the model, the QC+D' is assumed to be equal to QC+D in unperturbed conditions and remains constant regardless of the A22 concentration (Line 548-554). This assumption is reasonable if the minimum time interval for segregation (D') is irrelevant to the change of cell width. But how D' and QC+D' changes with cell width are unknown. Earlier molecular studies revealed that the polymerization of MreB affects the activity of topoisomerase IV, an enzyme mediates the dimerization of sister chromosomes, which implies that changing cell width may affect D'. Given the importance of QC+D' to the model, it is vital for the authors to make this assumption clear in maintext and explain why such assumption is reasonable.
-