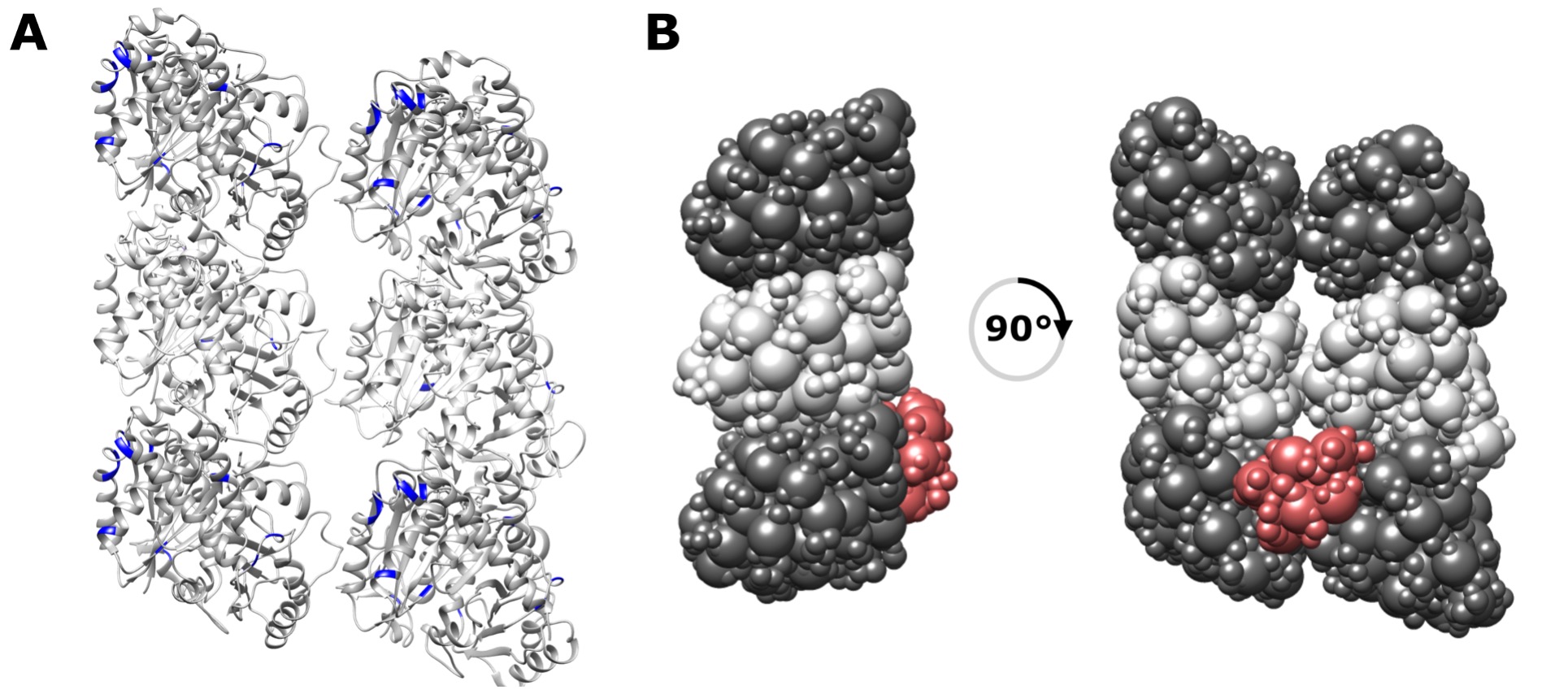
Doublecortin engages the microtubule lattice through a cooperative binding mode involving its C-terminal domain
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Rafiei et al. investigate the molecular architecture of an important neuronal microtubule-associated protein, doublecortin, bound to the microtubule by integrating data from chemical cross-linking experiments with available crystallographic and cryo-EM structures. They present an appealing model of microtubule-mediated self-association of doublecortin; however, they do not perform any additional assays to support the functional relevance of this model. In addition, there are limitations to the used method in resolving structural details. The manuscript will be relevant for biologists with an interest in microtubule formation and to researchers who apply different structural biology tools to study the organization of large biomolecular assemblies.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 and Reviewer #3 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Doublecortin (DCX) is a microtubule (MT)-associated protein that regulates MT structure and function during neuronal development and mutations in DCX lead to a spectrum of neurological disorders. The structural properties of MT-bound DCX that explain these disorders are incompletely determined. Here, we describe the molecular architecture of the DCX–MT complex through an integrative modeling approach that combines data from X-ray crystallography, cryo-electron microscopy, and a high-fidelity chemical crosslinking method. We demonstrate that DCX interacts with MTs through its N-terminal domain and induces a lattice-dependent self-association involving the C-terminal structured domain and its disordered tail, in a conformation that favors an open, domain-swapped state. The networked state can accommodate multiple different attachment points on the MT lattice, all of which orient the C-terminal tails away from the lattice. As numerous disease mutations cluster in the C-terminus, and regulatory phosphorylations cluster in its tail, our study shows that lattice-driven self-assembly is an important property of DCX.
Article activity feed
-

-

Author Response:
Reviewer #2 (Public Review):
The neuronal MAP doublecortin contains two homologous DC domains, referred to as NDC and CDC. Disease-causing mutations cluster in these domains and both have been implicated in microtubule binding. However, the stoichiometry of DCX:tubulin dimers on microtubules is 1:1, suggesting only one of these domains is DCX's primary microtubule binding module. Early structural studies by Kim et al, 2001, identified different properties of NDC and CDC, despite their predicted homology. High resolution structures of both NDC and CDC have since been determined using X-ray crystallography and NMR - the domains do adopt the same overall fold, although DCX CDC structures were determined either a) bound to nanobodies (Burger et al, 2016; 5IP4) or b) forming a domain swapped dimer in a protein purified at …
Author Response:
Reviewer #2 (Public Review):
The neuronal MAP doublecortin contains two homologous DC domains, referred to as NDC and CDC. Disease-causing mutations cluster in these domains and both have been implicated in microtubule binding. However, the stoichiometry of DCX:tubulin dimers on microtubules is 1:1, suggesting only one of these domains is DCX's primary microtubule binding module. Early structural studies by Kim et al, 2001, identified different properties of NDC and CDC, despite their predicted homology. High resolution structures of both NDC and CDC have since been determined using X-ray crystallography and NMR - the domains do adopt the same overall fold, although DCX CDC structures were determined either a) bound to nanobodies (Burger et al, 2016; 5IP4) or b) forming a domain swapped dimer in a protein purified at pH 10.5 (Rufer et al, 2018; 6FNZ).
The structures of microtubule-bound DCX have also been determined using cryo-EM - these show DCX's primary microtubule binding site is in the valley between protofilaments at the corner of four tubulin dimers. Most recently, the structures of full-length DCX at different microtubule polymerization time points have been captured at ~4A resolution (Manka & Moores, 2020). The structures of microtubule-bound CDC (6RF2) and microtubule-bound NDC (6REV) were thereby determined, but only a single DC domain at the DCX primary binding site has ever been observed.
Thus, despite the accumulated DCX structural data, a number of significant questions remain - notably, how is the full-length protein involved in binding to microtubules and what is the structural origin of the cooperative microtubule binding by DCX, which is mediated by CDC (Bechstedt and Brouhard, 2012)
Rafiei et al use an integrated structural modelling approach, synthesizing cross-linking mass spectrometry data of microtubule-bound DCX with existing structural information to provide new perspectives on DCX's microtubule binding mechanism. The particular strengths of this approach are that the data are both detailed, and capable of capturing the heterogeneity and dynamics of the system. The incorporation of prior structural knowledge into the workflow mean that these analyses sit alongside existing data, rather than being completely independent from them.
Overall, the authors confirm findings in the literature that NDC is DCX's primary microtubule binding domain for microtubules polymerized for >30 minutes. They also find that CDC mediates microtubule-binding dependent dimerization, which could explain DCX's cooperative behavior. There are several aspects of the study that would benefit from further analysis and/or discussion to clarify potential limitations of, or assumptions in, the approaches taken:
- Although the authors report that the crosslinker used in their mass-spec experiments has been optimized for use with microtubules, it is not clear how general DCX binding is in this context. Specifically, how accessible are the well-buried DCX-tubulin interfaces at the primary binding site to the chemical cross-linkers on which the analysis depends? Accessibility issues could explain the results depicted in Fig. 3A, B, in which modelling that relies strictly on cross-links places NDC towards the outer edge of the protofilament, whereas inclusion of cryo-EM data in the integrated model places NDC in the inter-protofilament valley.
There are no accessibility issues related to the crosslinks. In fact, we observe crosslinks to sites that are well buried in the cleft, as shown in the figure below (1A). This is in line with data from a previous paper on MT crosslinking (Legal et al., 2016). The appearance of the models sitting near the outer edge of the protofilament is due to how we chose to represent the system, and is an expected edge effect. It is approximately half of the actual binding site and so expected to compete. To illustrate that accessibility is not an issue, we re-clustered the models with a lower threshold (2 Å) to generate smaller major cluster (22% of the total) where the NDC is positioned even more deeply within the inter-protofilament valley, as shown in the figure below (1B). Clustering at higher threshold is preferred because it repesents modeling uncertainty more faithfully by including the majority of the models generated during sampling.
Figure 1 (A) Crosslink sites on the MT lattice repeat unit highlighted in blue, showing that some are indeed buried within the interprotofilament groove. (B) Alternative representation showing the buried nature of NDC on the lattice.
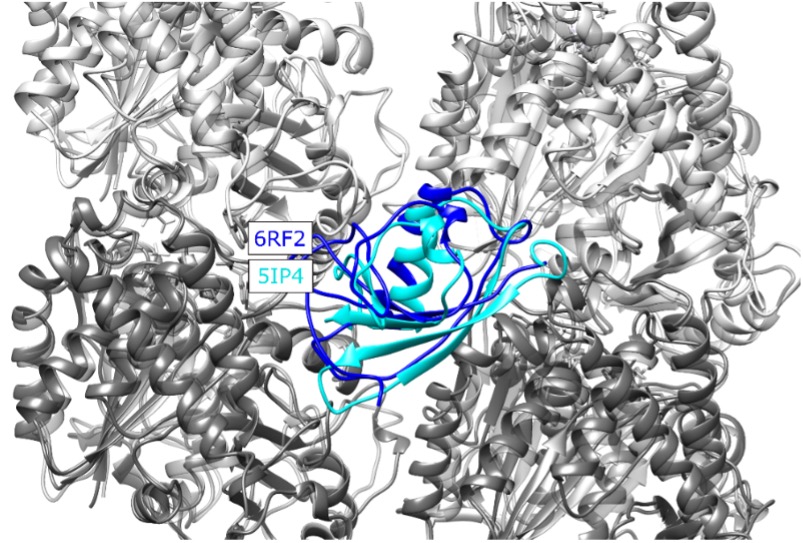
- Based on analysis using the nanobody-bound CDC structure (5IP4), CDC appears to behave distinctly compared to NDC, such that CDC-derived cross-linking data are not consistent with the canonical inter-protofilament binding site. It would be good know whether this depends on the particular PDB used. It would be important to repeat this analysis using the microtubule-bound structure of CDC (6RF2), given that this structure is conformationally distinct from PDB:5IP.
We calculate the RMSD between 5IP4 and 6RF2 to be 5.1 Å, and show the alignment of the structures below. This is a small difference when considering the precision of our integrative method, and thus would not change the results/conclusions presented in our paper. (Note that crosslinks are contrained with a distance of ~25 Å or less.) We have added a statement to the text to reflect this.
Figure 2 Structural alignment of the new MT-CDC structure (6RF2) to the one used in our study (5IP4), placed at the NDC binding site for illustration. CDC structures corresponding to 6RF2 and 5IP4 are shown with blue and cyan, respectively, alpha tubulins are shown in light grey and beta tubulins are shown in dark grey, The RMSD calculated for residues 178-251 of the 5IP4 and 6RF2 is 5.1 Å.
- Building on these findings relating to DCX-microtubule interactions, further analyses focus on DCX-DCX cross links, the formation of which are shown to be microtubule-dependent. The authors observe that >80% of DCX-DCX crosslinks involve the CDC domain and the C-terminus of the protein (C-tail), which is also consistent with NDC being the major point of microtubule interaction. However, a crucial aspect of this analysis is how readily microtubule-mediated oligomerization of DCX-DCX can be discriminated from the non-specific interactions that occur due to the high local concentrations on the microtubule surface. Given the proposed primary microtubule binding role of NDC, either set of interactions would presumably involve CDC and C-tail. Additional control experiments would have been beneficial here.
Although their data do not allow them to discriminate between different oligomerization states of DCX, the authors focus on dimer formation, and they interrogate their data based on interactions between CDC domains either i) retaining a globular fold or ii) adopting the "open" state seen in the 6FNZ domain-swapped dimer. According to the authors: "Based purely on fit of crosslinks, globular or domain-swapped modes are not distinguishable (Fig 4B). However, modelling of the main cluster shows strong similarity to the domain-swapped dimer structure"
This is a pivotal point of the manuscript. However, the precise quantitative basis of this discrimination is not clearly described. A useful control for these experiments could also be a previously published NDC-NDC chimera (Manka & Moores, 2020), which binds microtubules at the same inter-protofilament site but which lacks the CDC domain that is potentially mediating oligomerization.
The authors present an appealing model for CDC-mediated dimerisation of DCX on the microtubule lattice, but do not directly test its functional relevance. It will be crucial to explore the significance of dimer formation further. In the meantime, while questions concerning the mode of interaction of DCX (and its relatives) with the microtubule lattice are very much alive, the findings in the current study are not currently definitive.
We thank the reviewer for these insights. We note that nonspecific aggregation of DCX on the MT lattice is unlikely, given the absence of aggregation at high concentration in free solution, even under induced denaturation. Further, we would expect such aggregation to be far less localized than we observe. We hope that the addition of the R303X truncation and the TIRF-based cooperativity data provides additional confidence in our claim that lattice-driven self-association is an important element of DCX function.
-

Evaluation Summary:
Rafiei et al. investigate the molecular architecture of an important neuronal microtubule-associated protein, doublecortin, bound to the microtubule by integrating data from chemical cross-linking experiments with available crystallographic and cryo-EM structures. They present an appealing model of microtubule-mediated self-association of doublecortin; however, they do not perform any additional assays to support the functional relevance of this model. In addition, there are limitations to the used method in resolving structural details. The manuscript will be relevant for biologists with an interest in microtubule formation and to researchers who apply different structural biology tools to study the organization of large biomolecular assemblies.
(This preprint has been reviewed by eLife. We include the public …
Evaluation Summary:
Rafiei et al. investigate the molecular architecture of an important neuronal microtubule-associated protein, doublecortin, bound to the microtubule by integrating data from chemical cross-linking experiments with available crystallographic and cryo-EM structures. They present an appealing model of microtubule-mediated self-association of doublecortin; however, they do not perform any additional assays to support the functional relevance of this model. In addition, there are limitations to the used method in resolving structural details. The manuscript will be relevant for biologists with an interest in microtubule formation and to researchers who apply different structural biology tools to study the organization of large biomolecular assemblies.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 and Reviewer #3 agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
In their manuscript, Rafiei et al. investigate the molecular architecture of the microtubule-associated protein, doublecortin-X (DCX), bound to the microtubule by integrating data from chemical cross-linking experiments with available crystallographic and cryo-EM structures. DCX contains two doublecortin (DC) domains (termed N-DC and C-DC) connected by a linker region and an unstructured C-terminal tail. The DC domains have both been shown to be important for its binding to microtubules. Prior work has shown that DCX interacts with the GTP microtubule lattice through its C-DC and the GDP-lattice through its N-DC. In addition, it is well-established that DCX cooperatively binds the microtubule lattice, but how this cooperativity is achieved is still unclear. This study evaluates inter- and intra- DCX …
Reviewer #1 (Public Review):
In their manuscript, Rafiei et al. investigate the molecular architecture of the microtubule-associated protein, doublecortin-X (DCX), bound to the microtubule by integrating data from chemical cross-linking experiments with available crystallographic and cryo-EM structures. DCX contains two doublecortin (DC) domains (termed N-DC and C-DC) connected by a linker region and an unstructured C-terminal tail. The DC domains have both been shown to be important for its binding to microtubules. Prior work has shown that DCX interacts with the GTP microtubule lattice through its C-DC and the GDP-lattice through its N-DC. In addition, it is well-established that DCX cooperatively binds the microtubule lattice, but how this cooperativity is achieved is still unclear. This study evaluates inter- and intra- DCX crosslinking using a protein isotopic labeling technique. Combined with modeling, they are able to show that DCX binds to microtubules through its N-DC, which subsequently induces DCX to self-associates through its C-DC and C-terminal tail domains; however, they do not perform any additional biochemical, single-molecule, or ensemble binding assays to support this model. This paper will be of interest to the microtubule community.
-

Reviewer #2 (Public Review):
The neuronal MAP doublecortin contains two homologous DC domains, referred to as NDC and CDC. Disease-causing mutations cluster in these domains and both have been implicated in microtubule binding. However, the stoichiometry of DCX:tubulin dimers on microtubules is 1:1, suggesting only one of these domains is DCX's primary microtubule binding module. Early structural studies by Kim et al, 2001, identified different properties of NDC and CDC, despite their predicted homology. High resolution structures of both NDC and CDC have since been determined using X-ray crystallography and NMR - the domains do adopt the same overall fold, although DCX CDC structures were determined either a) bound to nanobodies (Burger et al, 2016; 5IP4) or b) forming a domain swapped dimer in a protein purified at pH 10.5 (Rufer et …
Reviewer #2 (Public Review):
The neuronal MAP doublecortin contains two homologous DC domains, referred to as NDC and CDC. Disease-causing mutations cluster in these domains and both have been implicated in microtubule binding. However, the stoichiometry of DCX:tubulin dimers on microtubules is 1:1, suggesting only one of these domains is DCX's primary microtubule binding module. Early structural studies by Kim et al, 2001, identified different properties of NDC and CDC, despite their predicted homology. High resolution structures of both NDC and CDC have since been determined using X-ray crystallography and NMR - the domains do adopt the same overall fold, although DCX CDC structures were determined either a) bound to nanobodies (Burger et al, 2016; 5IP4) or b) forming a domain swapped dimer in a protein purified at pH 10.5 (Rufer et al, 2018; 6FNZ).
The structures of microtubule-bound DCX have also been determined using cryo-EM - these show DCX's primary microtubule binding site is in the valley between protofilaments at the corner of four tubulin dimers. Most recently, the structures of full-length DCX at different microtubule polymerization time points have been captured at ~4A resolution (Manka & Moores, 2020). The structures of microtubule-bound CDC (6RF2) and microtubule-bound NDC (6REV) were thereby determined, but only a single DC domain at the DCX primary binding site has ever been observed.
Thus, despite the accumulated DCX structural data, a number of significant questions remain - notably, how is the full-length protein involved in binding to microtubules and what is the structural origin of the cooperative microtubule binding by DCX, which is mediated by CDC (Bechstedt and Brouhard, 2012)
Rafiei et al use an integrated structural modelling approach, synthesizing cross-linking mass spectrometry data of microtubule-bound DCX with existing structural information to provide new perspectives on DCX's microtubule binding mechanism. The particular strengths of this approach are that the data are both detailed, and capable of capturing the heterogeneity and dynamics of the system. The incorporation of prior structural knowledge into the workflow mean that these analyses sit alongside existing data, rather than being completely independent from them.
Overall, the authors confirm findings in the literature that NDC is DCX's primary microtubule binding domain for microtubules polymerized for >30 minutes. They also find that CDC mediates microtubule-binding dependent dimerization, which could explain DCX's cooperative behavior. There are several aspects of the study that would benefit from further analysis and/or discussion to clarify potential limitations of, or assumptions in, the approaches taken:
Although the authors report that the crosslinker used in their mass-spec experiments has been optimized for use with microtubules, it is not clear how general DCX binding is in this context. Specifically, how accessible are the well-buried DCX-tubulin interfaces at the primary binding site to the chemical cross-linkers on which the analysis depends? Accessibility issues could explain the results depicted in Fig. 3A, B, in which modelling that relies strictly on cross-links places NDC towards the outer edge of the protofilament, whereas inclusion of cryo-EM data in the integrated model places NDC in the inter-protofilament valley.
Based on analysis using the nanobody-bound CDC structure (5IP4), CDC appears to behave distinctly compared to NDC, such that CDC-derived cross-linking data are not consistent with the canonical inter-protofilament binding site. It would be good know whether this depends on the particular PDB used. It would be important to repeat this analysis using the microtubule-bound structure of CDC (6RF2), given that this structure is conformationally distinct from PDB:5IP.
Building on these findings relating to DCX-microtubule interactions, further analyses focus on DCX-DCX cross links, the formation of which are shown to be microtubule-dependent. The authors observe that >80% of DCX-DCX crosslinks involve the CDC domain and the C-terminus of the protein (C-tail), which is also consistent with NDC being the major point of microtubule interaction. However, a crucial aspect of this analysis is how readily microtubule-mediated oligomerization of DCX-DCX can be discriminated from the non-specific interactions that occur due to the high local concentrations on the microtubule surface. Given the proposed primary microtubule binding role of NDC, either set of interactions would presumably involve CDC and C-tail. Additional control experiments would have been beneficial here.
Although their data do not allow them to discriminate between different oligomerization states of DCX, the authors focus on dimer formation, and they interrogate their data based on interactions between CDC domains either i) retaining a globular fold or ii) adopting the "open" state seen in the 6FNZ domain-swapped dimer. According to the authors: "Based purely on fit of crosslinks, globular or domain-swapped modes are not distinguishable (Fig 4B). However, modelling of the main cluster shows strong similarity to the domain-swapped dimer structure"
This is a pivotal point of the manuscript. However, the precise quantitative basis of this discrimination is not clearly described. A useful control for these experiments could also be a previously published NDC-NDC chimera (Manka & Moores, 2020), which binds microtubules at the same inter-protofilament site but which lacks the CDC domain that is potentially mediating oligomerization.
The authors present an appealing model for CDC-mediated dimerisation of DCX on the microtubule lattice, but do not directly test its functional relevance. It will be crucial to explore the significance of dimer formation further. In the meantime, while questions concerning the mode of interaction of DCX (and its relatives) with the microtubule lattice are very much alive, the findings in the current study are not currently definitive.
-

Reviewer #3 (Public Review):
Rafiei et al. use crosslinking mass spectrometry and integrative/hybrid structural modeling to study the interaction of the regulatory protein, doublecortin (DCX), with microtubules. Specifically, they use a heterobifunctional, amine-reactive and photoreactive crosslinking reagent to generate residue-level contacts within DCX and between DCX and the alpha- and beta-tubulin subunits in the microtubules. The authors show that because of the short timescale of the crosslink formation, this approach proves superior when compared to conventional solution crosslinking chemistries such as those using homobifunctional succinimide esters. The crosslinking restraints are subsequently used to provide a model for the interaction of DCX with mictotubules at domain level resolution. Finally, the resulting model is …
Reviewer #3 (Public Review):
Rafiei et al. use crosslinking mass spectrometry and integrative/hybrid structural modeling to study the interaction of the regulatory protein, doublecortin (DCX), with microtubules. Specifically, they use a heterobifunctional, amine-reactive and photoreactive crosslinking reagent to generate residue-level contacts within DCX and between DCX and the alpha- and beta-tubulin subunits in the microtubules. The authors show that because of the short timescale of the crosslink formation, this approach proves superior when compared to conventional solution crosslinking chemistries such as those using homobifunctional succinimide esters. The crosslinking restraints are subsequently used to provide a model for the interaction of DCX with mictotubules at domain level resolution. Finally, the resulting model is interpreted in view of a proposed mechanism for microtubule nucleation and stabilization.
The study uses crosslinking to study a highly challenging system, i.e. the binding of a multi-domain regulatory protein (that itself homodimerizes) to an extended tubular structure, the microtubule, in what is probably a dynamic interaction. DCX consists of two ordered domains connected and flanked by unstructured regions, resulting in a lot of flexibility in the molecule and many possible domain orientations within the dimer and with the microtubule. Crosslinking, in isolation, is typically not the technique that one would consider ideally suitable to characterize such a system. However, the authors could successfully and convincingly demonstrate that using an optimized crosslinking strategy in combination with integrative modeling, deeper insights into the architecture of such an assembly can be obtained.
Given the limited resolution of the crosslinking restraints, the obtained experimental data does not allow for a exhaustive description of the structure of the system. For example, the location of the N-terminal DCX domain can more precisely determined so that it may be concluded that this domain is the primary interaction site, while the location of the C-terminal domain remains more ambiguous. As for the self-assembly of DCX, no clear preference of any orientation of the monomer units in the dimer is observed. This is not a deficiency of the work per se, but only reflects the relatively low accuracy distance restraints.
In summary, I consider this a very interesting application with impact on structural and functional biology.
-