Spatial modulation of individual behaviors enables an ordered structure of diverse phenotypes during bacterial group migration
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The authors present a study on the cohesion maintenance of E.coli during collective migration in a self-generated gradient. They performed experiments and complemented the study with a predictive model and simulation to understand how bacteria with different phenotype are able to move as a cohesive group and how the individual bacterium defines its own position within the group. Particularly interesting aspects of the study are the use of titration of behavior with chemoreceptor abundance and the use of potential wells to model the attraction of bacteria to the center of their cohesive group. This approach will be of interest to physicists and biologists interested in collective motility and migration.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript.The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Coordination of diverse individuals often requires sophisticated communications and high-order computational abilities. Microbial populations can exhibit diverse individualistic behaviors, and yet can engage in collective migratory patterns with a spatially sorted arrangement of phenotypes. However, it is unclear how such spatially sorted patterns emerge from diverse individuals without complex computational abilities. Here, by investigating the single-cell trajectories during group migration, we discovered that, despite the constant migrating speed of a group, the drift velocities of individual bacteria decrease from the back to the front. With a Langevin-type modeling framework, we showed that this decreasing profile of drift velocities implies the spatial modulation of individual run-and-tumble random motions, and enables the bacterial population to migrate as a pushed wave front. Theoretical analysis and stochastic simulations further predicted that the pushed wave front can help a diverse population to stay in a tight group, while diverse individuals perform the same type of mean reverting processes around centers orderly aligned by their chemotactic abilities. This mechanism about the emergence of orderly collective migration from diverse individuals is experimentally demonstrated by titration of bacterial chemoreceptor abundance. These results reveal a simple computational principle for emergent ordered behaviors from heterogeneous individuals.
Article activity feed
-

Author Response
Reviewer #1 (Public Review):
In this paper, Bai et al. investigate in experiments and simulations how cohesion is maintained in chemotactic travelling waves of bacteria. These waves emerge from the bacterial population consuming an attractant, thus carving a gradient which they follow chemotactically. This paper builds up on previous work of some of the authors (Fu et al, Nat Commun 2018), which found that in these waves bacteria with varying degree of chemotactic sensitivity organize spatially in the band, which allows for its cohesiveness despite varying phenotypes. The authors investigate here an additional element for the cohesiveness of the wave: because the sharpness of the gradient increases from the front to the back of the wave, 'late' cells catch up via a stronger chemotactic response, and front cells slow …
Author Response
Reviewer #1 (Public Review):
In this paper, Bai et al. investigate in experiments and simulations how cohesion is maintained in chemotactic travelling waves of bacteria. These waves emerge from the bacterial population consuming an attractant, thus carving a gradient which they follow chemotactically. This paper builds up on previous work of some of the authors (Fu et al, Nat Commun 2018), which found that in these waves bacteria with varying degree of chemotactic sensitivity organize spatially in the band, which allows for its cohesiveness despite varying phenotypes. The authors investigate here an additional element for the cohesiveness of the wave: because the sharpness of the gradient increases from the front to the back of the wave, 'late' cells catch up via a stronger chemotactic response, and front cells slow down via a weaker one. This had been already postulated in earlier work on the phenomenon (Saragosti et al. PNAS 2011), but here the authors investigate how this applies to cells with varying chemotactic sensitivity. They also performed agent-based simulations of the cells behavior in the gradient and developed a model of the motion in the gradient. The latter maps the spatial dependence of the gradient steepness onto an effective travelling potential which keeps the cells together in a group as the gradient and the wave propagate. Importantly, the effective potential is predicted to be tighter for cells with higher chemotactic sensitivity, in agreement with the cell behavior they observe in experiments where the chemotactic sensitivity is artificially modulated. This suggests that weakly chemotactic cells are more weakly bound to the group and have a higher chance of being left behind. This last part is interesting in the context of range extension in semi-solid agar, where bacteria are known to be spatially organized and selected according to their chemotactic motility (Ni et al, Cell reports 2017, Liu et al Nature 2019)
This paper builds its strengths on the extensive experimental characterization of the system and a variety of modeling approaches and makes a fairly convincing case for the way of understanding the mechanism of cohesion maintenance they propose.
In fact, we have addressed both the mechanism to maintain a coherent group and also the mechanism to form ordered pattern of diverse phenotypes. Thanks to the reviewer, we noticed that the second point was not clearly showed out in our previous version. So that we have largely rewritten the texts and reorganized the results to prominent both mechanism.
From a methodological perspective, only a few points need to be addressed:
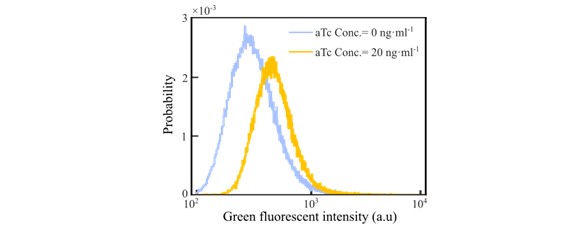
Control experiments need to quantify the cell-to-cell variability of the induction level of Tar by tetracycline.
The distributions of the titrate cells are presented by a ptet-Tar-GFP strain, where the GFP is used as a reporter of the expressed Tar protein. The results are shown below:
Chemical attraction to cues released by other cells is a well-documented way to create cohesive large scale structures in E. coli (Budrene & Berg Nature 1995, Park et al PNAS 2003, Jani et al Microbiology 2017, Laganenka et al Nat commun 2016). The cohesion of the wave have never been analyzed in this optic, despite being a possible alternative explanation to the gradient shape. Since the authors main claim is about the wave cohesion, they should provide evidence that such an explanation can be ruled out or considered secondary.
We thank the reviewer to point out the self-attractant secretion as a possible mechanism to maintain coherent group. We argue that this mechanism is not necessary for the chemotactic group to maintain coherency, because the migration group keeps without considering these effect in our agent based simulations.
Moreover, as suggested by the reviewer, we Used a Tar only strain, which do not sense any chemo-attractant other than aspartate, to show that the migration group maintained coherent (see Fig S9). This experiment showed that the secretion of self-attractant is not essential for the coherent group migration.
Possible effects of physical interactions between cells on the chemotactic response are not accounted for. The consequences should be better discussed, because they are known to influence chemotactic motility at the densities encountered in the present experiments (Colin et al Nat commun 2019).
As being reported by Colin et al., the effective drift velocity and the chemotactic ability deceases when cells are condensed (volume fraction >0.01). However, the cell density is smaller than this critical value (volume fraction<0.01).
Additionally, the paper could better emphasize the new results and separate them from the confirmations of previous results.
In the revised version, we addressed 2 new findings:
The individual drift velocity decreases from back to front of the bacterial migration group, which makes the chemotactic migration wave a pushed wave.
Cells of diversed phenotypes follows the same reversion behavior, ie. drift faster in the back and slower in the front, but with ordered mean positions, to achieve the ordered pattern in the migration group.
Reviewer #2 (Public Review):
The manuscript by Bai et al. explores the single-cell motility dynamics within a chemotactic soliton wave in E. coli. They tracked individual cells and measured their trajectory speed and orientation distributions behind and ahead of the wave. They showed cells behind the wave were moving in a more directed fashion towards the center of the wave compared to cells ahead of the wave. This behavior explains the stability of group migration, as confirmed by numerical simulations.
I do not recommend this manuscript for publication in eLife since it basically reproduces and deepens previous published works. In particular, Saragosti et al (2011) already provided exactly what the authors claim to do here : "How individuals with phenotypic and behavioral variations manage to maintain the consistent group performance and determine their relative positions in the group is still a mystery." (Line 75-77) (See the last sentences from Saragosti et al : "This modulation of the reorientations significantly improves the efficiency of the collective migration. Moreover, these two quantities are spatially modulated along the concentration profile. We recover quantitatively these microscopic and macroscopic observations with a dedicated kinetic model.")
Saragosti et al.talks about the modulation of reorientation angle of bacteria along directions. It is not equal to the spatial modulation of drift velocities along space. They claim that cells moving along the gradient direction reorient less during a tumble than cells moving against the gradient. This phenomenon increases the migration efficiency of the group. Here, in our paper, we claim that the drift velocity of bacteria is spatially modulated, where cells on the back drifts faster while the cells in the front drift slower. This phenomenon is important because it makes the chemotactic migration front a pushed wave, that helps the group to keep diversed phenotypes.
Although Saragosti et al. Have also suggested spatial modulation of bias in run length to explain the coherency of the migration group. But they did not quantify such bias nor did they explain the causes and consequences of the spatial modulation. More over, Their model, consisting their proposed mechanism of directional persistence, can not explain their observed phenomenon of the decreasing bias of run length (see their figure 4A and C).In this circumstance, we can’t agree that they already proofed how cells with diversed phenotype to maintain coherent group.
Moreover, they did not talk about diversities in the group.
What is novel here is the titration of the behavior with chemo-receptor abundance, but I believe the scope is not wide enough for publication in eLife. I suggest the authors to submit in a more specialized journal.
The titration of the chemo-receptor abundance of bacteria serves as a tool to explain how diverse individuals manage to form the ordered patterns in a group. This question worth several discussion because diversity is known as an important feature to keep a group to survive. The ordered pattern was found the key for a migrating group to keep the diversity while performing consistent migration speed. In this paper we successfully explained how individuals performing biased random walk are able to form ordered structure.
Reviewer #3 (Public Review):
The authors present a study on the collective behaviour of E.coli during migration in a self-generated gradient. Taking into account phenotypic variation within a biological population, they performed experiments and complemented the study with a predictive model used for simulation to understand how bacteria can move as a group and how the individual bacterium defines its own position within the group.
They observed experimentally that phenotype variation within the bacterial population causes a spatial distribution within the chemotactic band that is not continuous but formed by subpopulations with specific properties such as run length, run duration, angular distribution of trajectories, drift velocity. They attribute this behaviour to the chemotaxis ability, which varies between phenotypes and defines a potential well that anchors each bacterium in its own group. This was proven by the subdiffusive dynamics of the bacteria in each subgroup. Many cases were studied in the experiments and the authors present many controls to clearly demonstrate their hypothesis.
These are interesting results that prove how a discretised distribution can produce continuous collective behaviour. It presents also an interesting example in the field of active matter about collective behaviour on a large scale that is generated by a different behaviour of individuals on a much smaller scale. However, it is not clear how the subpopulations can be held together in the group.
The decreasing chemo-attractant gradient makes the migration wavefront a pushed wavefront. So that the balanced position of the subpopulation with larger chemotactic ability is located in the front where the gradient is small. So that diverse phenotypes form ordered pattern to achieve identical migration speed on their balanced positions. This discussion was added in the revised text (see line 268-277).
Moreover, a link between bacterial dynamics and the biological necessary mechanism is not clear.
The bacterial individual dynamics is controlled by the bacterial chemotaxis pathway, which is clear according to previous studies. Basically, the biased random motion was controlled by alternating expected run length through a temporal comparison mechanism between received chemo-attractant concentrations.(Jiang et al. 2010 Plos Comp. Biol.)
They formulate a theoretical description based on the classical Keller-Segel model. Langevin dynamics was used to describe bacterial activity in terms of drift velocity for simulation, which agrees very well with experimental observations.
One can appreciate the interesting results of the study describing Ecoli chemotaxis as a mean-reversion process with an associated potential, but it is not clear to what extent the results can be generalised to all bacteria or rather relate to the strain the authors investigated.
The mean reversion process is a result of decreasing drift velocity (or a pushed wave). Although our study focuses on bacterail chemotaxis migration, but the ordering mechanism of diversed phenotypes follows a OU type model, which is not limited to bacterial chemotaxis. In this case, we argue that the ordering mechanism that we proposed is universal to all active particles that generate signals as a global cue of collective motion.
-

Evaluation Summary:
The authors present a study on the cohesion maintenance of E.coli during collective migration in a self-generated gradient. They performed experiments and complemented the study with a predictive model and simulation to understand how bacteria with different phenotype are able to move as a cohesive group and how the individual bacterium defines its own position within the group. Particularly interesting aspects of the study are the use of titration of behavior with chemoreceptor abundance and the use of potential wells to model the attraction of bacteria to the center of their cohesive group. This approach will be of interest to physicists and biologists interested in collective motility and migration.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also …
Evaluation Summary:
The authors present a study on the cohesion maintenance of E.coli during collective migration in a self-generated gradient. They performed experiments and complemented the study with a predictive model and simulation to understand how bacteria with different phenotype are able to move as a cohesive group and how the individual bacterium defines its own position within the group. Particularly interesting aspects of the study are the use of titration of behavior with chemoreceptor abundance and the use of potential wells to model the attraction of bacteria to the center of their cohesive group. This approach will be of interest to physicists and biologists interested in collective motility and migration.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript.The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
In this paper, Bai et al. investigate in experiments and simulations how cohesion is maintained in chemotactic travelling waves of bacteria. These waves emerge from the bacterial population consuming an attractant, thus carving a gradient which they follow chemotactically. This paper builds up on previous work of some of the authors (Fu et al, Nat Commun 2018), which found that in these waves bacteria with varying degree of chemotactic sensitivity organize spatially in the band, which allows for its cohesiveness despite varying phenotypes. The authors investigate here an additional element for the cohesiveness of the wave: because the sharpness of the gradient increases from the front to the back of the wave, 'late' cells catch up via a stronger chemotactic response, and front cells slow down via a weaker …
Reviewer #1 (Public Review):
In this paper, Bai et al. investigate in experiments and simulations how cohesion is maintained in chemotactic travelling waves of bacteria. These waves emerge from the bacterial population consuming an attractant, thus carving a gradient which they follow chemotactically. This paper builds up on previous work of some of the authors (Fu et al, Nat Commun 2018), which found that in these waves bacteria with varying degree of chemotactic sensitivity organize spatially in the band, which allows for its cohesiveness despite varying phenotypes. The authors investigate here an additional element for the cohesiveness of the wave: because the sharpness of the gradient increases from the front to the back of the wave, 'late' cells catch up via a stronger chemotactic response, and front cells slow down via a weaker one. This had been already postulated in earlier work on the phenomenon (Saragosti et al. PNAS 2011), but here the authors investigate how this applies to cells with varying chemotactic sensitivity. They also performed agent-based simulations of the cells behavior in the gradient and developed a model of the motion in the gradient. The latter maps the spatial dependence of the gradient steepness onto an effective travelling potential which keeps the cells together in a group as the gradient and the wave propagate. Importantly, the effective potential is predicted to be tighter for cells with higher chemotactic sensitivity, in agreement with the cell behavior they observe in experiments where the chemotactic sensitivity is artificially modulated. This suggests that weakly chemotactic cells are more weakly bound to the group and have a higher chance of being left behind. This last part is interesting in the context of range extension in semi-solid agar, where bacteria are known to be spatially organized and selected according to their chemotactic motility (Ni et al, Cell reports 2017, Liu et al Nature 2019)
This paper builds its strengths on the extensive experimental characterization of the system and a variety of modeling approaches and makes a fairly convincing case for the way of understanding the mechanism of cohesion maintenance they propose.
From a methodological perspective, only a few points need to be addressed:Control experiments need to quantify the cell-to-cell variability of the induction level of Tar by tetracycline.
Chemical attraction to cues released by other cells is a well-documented way to create cohesive large scale structures in E. coli (Budrene & Berg Nature 1995, Park et al PNAS 2003, Jani et al Microbiology 2017, Laganenka et al Nat commun 2016). The cohesion of the wave have never been analyzed in this optic, despite being a possible alternative explanation to the gradient shape. Since the authors main claim is about the wave cohesion, they should provide evidence that such an explanation can be ruled out or considered secondary.
Possible effects of physical interactions between cells on the chemotactic response are not accounted for. The consequences should be better discussed, because they are known to influence chemotactic motility at the densities encountered in the present experiments (Colin et al Nat commun 2019).
Additionally, the paper could better emphasize the new results and separate them from the confirmations of previous results.
-

Reviewer #2 (Public Review):
The manuscript by Bai et al. explores the single-cell motility dynamics within a chemotactic soliton wave in E. coli. They tracked individual cells and measured their trajectory speed and orientation distributions behind and ahead of the wave. They showed cells behind the wave were moving in a more directed fashion towards the center of the wave compared to cells ahead of the wave. This behavior explains the stability of group migration, as confirmed by numerical simulations.
Saragosti et al (2011) already provided exactly what the authors claim to do here : "How individuals with phenotypic and behavioral variations manage to maintain the consistent group performance and determine their relative positions in the group is still a mystery." (Line 75-77) (See the last sentences from Saragosti et al : "This …
Reviewer #2 (Public Review):
The manuscript by Bai et al. explores the single-cell motility dynamics within a chemotactic soliton wave in E. coli. They tracked individual cells and measured their trajectory speed and orientation distributions behind and ahead of the wave. They showed cells behind the wave were moving in a more directed fashion towards the center of the wave compared to cells ahead of the wave. This behavior explains the stability of group migration, as confirmed by numerical simulations.
Saragosti et al (2011) already provided exactly what the authors claim to do here : "How individuals with phenotypic and behavioral variations manage to maintain the consistent group performance and determine their relative positions in the group is still a mystery." (Line 75-77) (See the last sentences from Saragosti et al : "This modulation of the reorientations significantly improves the efficiency of the collective migration. Moreover, these two quantities are spatially modulated along the concentration profile. We recover quantitatively these microscopic and macroscopic observations with a dedicated kinetic model.")
What is novel here is the titration of the behavior with chemo-receptor abundance.What is novel here is the titration of the behavior with chemo-receptor abundance, but I believe the scope is not wide enough for publication in eLife. I suggest the authors to submit in a more specialized journal.
-

Reviewer #3 (Public Review):
The authors present a study on the collective behaviour of E.coli during migration in a self-generated gradient. Taking into account phenotypic variation within a biological population, they performed experiments and complemented the study with a predictive model used for simulation to understand how bacteria can move as a group and how the individual bacterium defines its own position within the group.
They observed experimentally that phenotype variation within the bacterial population causes a spatial distribution within the chemotactic band that is not continuous but formed by subpopulations with specific properties such as run length, run duration, angular distribution of trajectories, drift velocity. They attribute this behaviour to the chemotaxis ability, which varies between phenotypes and defines a …
Reviewer #3 (Public Review):
The authors present a study on the collective behaviour of E.coli during migration in a self-generated gradient. Taking into account phenotypic variation within a biological population, they performed experiments and complemented the study with a predictive model used for simulation to understand how bacteria can move as a group and how the individual bacterium defines its own position within the group.
They observed experimentally that phenotype variation within the bacterial population causes a spatial distribution within the chemotactic band that is not continuous but formed by subpopulations with specific properties such as run length, run duration, angular distribution of trajectories, drift velocity. They attribute this behaviour to the chemotaxis ability, which varies between phenotypes and defines a potential well that anchors each bacterium in its own group. This was proven by the subdiffusive dynamics of the bacteria in each subgroup. Many cases were studied in the experiments and the authors present many controls to clearly demonstrate their hypothesis.
These are interesting results that prove how a discretised distribution can produce continuous collective behaviour. It presents also an interesting example in the field of active matter about collective behaviour on a large scale that is generated by a different behaviour of individuals on a much smaller scale. However, it is not clear how the subpopulations can be held together in the group. Moreover, a link between bacterial dynamics and the biological necessary mechanism is not clear.
They formulate a theoretical description based on the classical Keller-Segel model. Langevin dynamics was used to describe bacterial activity in terms of drift velocity for simulation, which agrees very well with experimental observations.
One can appreciate the interesting results of the study describing Ecoli chemotaxis as a mean-reversion process with an associated potential, but it is not clear to what extent the results can be generalised to all bacteria or rather relate to the strain the authors investigated.
-

-