Enhanced Cas12a multi-gene regulation using a CRISPR array separator
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This manuscript is of broad interest to those performing multiplexed genome engineering and related applications with CRISPR Cas12a technologies. While the proposed use of synSeparators is promising, the paper would benefit from further investigation of the mechanism by which synSeparators function to promote Cas12a activity. Additional data would be required to support the current conclusions regarding the generalizability of the findings.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The type V-A Cas12a protein can process its CRISPR array, a feature useful for multiplexed gene editing and regulation. However, CRISPR arrays often exhibit unpredictable performance due to interference between multiple guide RNA (gRNAs). Here, we report that Cas12a array performance is hypersensitive to the GC content of gRNA spacers, as high-GC spacers can impair activity of the downstream gRNA. We analyze naturally occurring CRISPR arrays and observe that natural repeats always contain an AT-rich fragment that separates gRNAs, which we term a CRISPR separator . Inspired by this observation, we design short, AT-rich synthetic separators ( synSeparators ) that successfully remove the disruptive effects between gRNAs. We further demonstrate enhanced simultaneous activation of seven endogenous genes in human cells using an array containing the synSeparator. These results elucidate a previously underexplored feature of natural CRISPR arrays and demonstrate how nature-inspired engineering solutions can improve multi-gene control in mammalian cells.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
The authors interrogated an underexplored feature of CRISPR arrays to enhance multiplexed genome engineering with the CRISPR nuclease Cas12a. Multiplexing represents one of the many desirable features of CRISPR technologies, and use of highly compact CRISPR arrays from CRISPR-Cas systems allows targeting of many sites at one time. Recent work has shown though that the composition of the array can have a major impact on the performance of individual guide RNAs encoded within the array, providing ample opportunities for further improvements. In this manuscript, the authors found that the region within the repeat lost through processing, what they term the separator, can have a major impact on targeting performance. The effect was specifically tied to upstream guide sequences with high GC …
Author Response:
Reviewer #1 (Public Review):
The authors interrogated an underexplored feature of CRISPR arrays to enhance multiplexed genome engineering with the CRISPR nuclease Cas12a. Multiplexing represents one of the many desirable features of CRISPR technologies, and use of highly compact CRISPR arrays from CRISPR-Cas systems allows targeting of many sites at one time. Recent work has shown though that the composition of the array can have a major impact on the performance of individual guide RNAs encoded within the array, providing ample opportunities for further improvements. In this manuscript, the authors found that the region within the repeat lost through processing, what they term the separator, can have a major impact on targeting performance. The effect was specifically tied to upstream guide sequences with high GC content. Introducing synthetic separator sequences shorter than their natural counterparts but exhibiting similarly low GC content boosted targeted activation of a reporter in human cells. Applying one synthetic separator to a seven-guide array targeting chromosomal genes led to consistent though more modest targeted activation. These findings introduce a distinct design consideration for CRISPR arrays that can further enhance the efficacy of multiplexed applications. The findings also suggest a selective pressure potentially influencing the repeat sequence in natural CRISPR arrays.
Strengths:
The portion of the repeat discarded through processing normally has been included or discarded when generating a CRISPR-Cas12a array. The authors clearly show that something in between-namely using a short version with a similarly low GC content-can enhance targeting over the truncated version. A coinciding surprising result was that the natural separator completely eliminated any measurable activation, necessitating the synthetic separator.
The manuscript provides a clear progression from identifying a feature of the upstream sequences impacting targeting to gaining insights from natural CRISPR-Cas12a systems to applying the insights to enhance array performance.
With further support, the use of synthetic separators could be widely adopted across the many applications of CRISPR-Cas12a arrays.
Weaknesses:
The terminology used to describe the different parts of the CRISPR array could better align with those in the CRISPR biology field. For one, crRNAs (abbreviated from CRISPR RNAs) should reflect the final processed form of the guide RNA, whereas guide RNAs (gRNAs) captures both pre-processed and post-processed forms. Also, "spacers" should reflect the natural spacers acquired by the CRISPR-Cas system, whereas "guides" better capture the final sequence in the gRNA used for DNA target recognition.
We thank the reviewer for this correction. We have now changed most uses of “crRNA” to “gRNA”. We decided to retain the use of the word “spacer” for the target recognition portion of the gRNA rather than changing it to “guide” as the reviewer suggests, because we think there is a risk that the reader would confuse “guide” with the non-synonymous “guide-RNA”. We have added a remark explaining our use of “spacer” (“A gRNA consists of a repeat region, which is often identical for all gRNAs in the array, and a spacer (here used synonymously with “guide region”)”)
A running argument of the work is that the separator specifically evolved to buffer adjacent crRNAs. However, this argument overlooks two key aspects of natural CRISPR arrays. First, the spacer (~30 nts) is normally much longer than the guide used in this work (20 nts), already providing the buffer described by the authors. This spacer also undergoes trimming to form the mature crRNA.
If we understand this comment correctly, the argument is that, in contrast to a ~20-nt spacer, a 30-nt spacer would provide a buffer between adjacent guides even if a separator is not present. However, even a 30-nt spacer may have high GC content and form secondary structures that would interfere with processing of the subsequent gRNA. Our hypothesis is that the separator is AT-rich and so insulates gRNAs from one another regardless of the length or GC composition of spacers. Please let us know if we have misunderstood this comment.
Second, the repeat length is normally fixed as a consequence of the mechanisms of spacer acquisition. At most, the beginning of each repeat sequence may have evolved to reduce folding interactions without changing the repeat length, although some of these repeats are predicted to fold into small hairpins.
We agree with this comment. Indeed, we propose that the separator, which is part of the repeat sequence, has evolved to reduce folding interactions. We now clarify this at the end of the Results section: “Taken together, the results from our study suggest that the CRISPR-separator has evolved as an integral part of the repeat region that likely insulates gRNAs from the disrupting effects of varying GC content in upstream spacers.”
Prior literature has highlighted the importance of a folded hairpin with an upstream pseudoknot within the repeat (Yamano Cell 2016), where disrupting this structure compromises DNA targeting by Cas12a (Liao Nat Commun 2019, Creutzburg NAR 2020). This structure is likely central to the authors' findings and needs to be incorporated into the analyses.
We thank the reviewer for this important insight. We have now performed experiments exploring the involvement of the pseudoknot in the disruptive effects of high-GC spacers.
First, we used our 2-gRNA CRISPR array design (Fig. 1D) where the second gRNA targets the GFP promoter and the first gRNA contains a non-targeting dummy spacer. We generated several versions of this array where we iteratively introduced targeted point mutations in the dummy spacer to either form a hairpin restricted to the dummy spacer, or a hairpin that would compete with the pseudoknot in the GFP-gRNA’s repeat region (new Fig. S3). We found that both of these modifications significantly reduced performance of the GFP-targeting gRNA. These results suggest that interfering with the pseudoknot indeed disrupts gRNA performance, but that also hairpins that presumably don’t interfere directly with the pseudoknot are detrimental – perhaps by sterically hindering Cas12a from accessing its cleavage site. Interestingly, the AAAT synSeparator largely rescued performance of the worst-performing of these constructs. These results are displayed in the new Fig. S3 and discussed in the related part of the Results section.
Second, we have now performed a computational analysis using RNAfold where we correlated the performance of all dummy spacers with their predicted secondary structure (Fig. 1M). The correlation between predicted RNA structure and array performance was higher when the structural prediction included both the dummy spacer and the entire GFP-targeting gRNA (R2 = 0.57) than when it included only the dummy spacer (R2 = 0.27; new figure panel S1C). This higher correlation suggests that secondary structures that involve the GFP-targeting gRNA play a more important role in our experiment than secondary structures that only involve the dummy spacer. These results are described in the Results section and in the Fig. 1 legend.
Third, we now also performed secondary structure analysis (RNAfold) of two of our worst-performing dummy spacers (50% and 70% GC), which indicated that these spacers are likely to form secondary structures that involve both the repeat and spacer of the downstream GFP-targeting gRNA (Fig. 3G-H). Interestingly, this analysis suggested that the AAAT synSeparator improves performance of these spacers by loosening up these secondary structures or creating an unstructured bulge at the Cas12a cleavage site. These results are presented in Fig. 3G-H and the accompanying portion of the Results section.
To conclude, our analyses suggest that the secondary structure in the spacer and its interference with the pseudoknot in the repeat hairpin play a role in gRNA performance, wherein the inclusion of the AAAT synSeparator can partly rescue the performance, likely by restoring the Cas12a accessibility to the gRNA cleavage site.
Many claims could better reflect the cited literature. For instance, Creutzburg et al. showed that adding secondary structures to the guide to promote folding of the repeat hairpin enhanced rather than interfered with targeting.
We thank the reviewer for this comment. Creutzburg et al. report the interesting finding that a carefully designed 3’ extension of the spacer can counteract secondary structures that disrupt the repeat. In this way, the extension rescues disruptive secondary structures that involve the repeat and any upstream sequence. Relevant to this finding, it is conceivable that the synSeparator (AAAT) exerts its beneficial effect at the 3’ end of the GFP spacer by folding back onto the GFP spacer and in this way blocking secondary structures caused by a GC-rich dummy spacer located upstream of the GFP gRNA, according to the mechanism reported by Creutzburg et al. However, we used structural prediction of the GFP-targeting gRNA with and without the AAAT synSeparator and did not find evidence that the AAAT extension would cause this spacer to fold back onto itself (data not shown). Moreover, our experimental data (Fig. 3E) demonstrate that the synSeparator exerts its main beneficial effect when located upstream of the GFP-targeting gRNA, which would not be the case if the main mechanism was the one demonstrated by Creutzburg et al. We already had a paragraph discussing the Creutzburg paper in the Discussion, but we have now added a sentence specifying the mechanism that Creutzburg et al. demonstrated: “RNA secondary structure prediction (RNAfold) did not indicate that the GFP-targeting spacer would fold back on itself when an AAAT extension is added to the 3’ end, which would have been the case for the mechanism demonstrated by Creutzburg et al. (data not shown).”
Liu et al. NAR 2019 further showed that the pre-processed repeat actually enhanced rather than reduced performance compared to the processed repeat.
The experiment referenced by the reviewer (Fig. 2 in Liu et al., Nucleic Acids Research, 2019) in fact nicely supports our findings. In Liu et al., the pre-processed repeat only shows improved performance if it is located upstream of the targeting gRNA, and the gRNA is not followed by an additional pre-processed repeat (DRf-crRNA in their Fig. 2B & C). In this situation, the pre-processed repeat (containing the natural separator) may serve to enhance gRNA processing, as would be expected based on our results. At the same time, the absence of a full-length repeat downstream of the gRNA means that after gRNA processing, there will not remain any piece of RNA attached to the 3’ end of the spacer, which might disrupt gRNA performance. In contrast, when Liu et al. added an additional pre-processed repeat downstream of their gRNA (DRf-crRNA-DRf in the same panel), this construct performed the worst of all tested variants. This is consistent with our conclusion that the full-length separator reduces performance of gRNAs if it remains attached to the 3’ end of spacers. We have added a paragraph in the Discussion about this (Line 376).
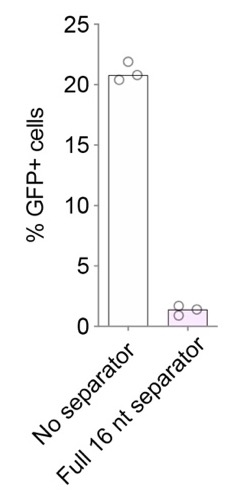
Finally, the complete loss of targeting with the unprocessed repeat appears represent an extreme example given multiple studies that showed effective targeting with this repeat (e.g. Liu NAR 2019, Zetsche Nat Biotechnol 2016).
We acknowledge that our CRISPR array containing the full, natural separator (Fig. 3B) appears to be completely non-functional in contrast to the studies mentioned by the reviewer. We think this difference may have a few possible explanations. First, this array is in fact not entirely non-functional. Re-running the same experiment with a stronger dCas12a-activator (dCas12a-VPR, full length VPR, also used in Fig. 5) shows some modest GFP activation even with the full separator (1.4% vs 20.8% GFP+ cells; see the Appendix Figure 1). But for consistency, we have used the same, slightly less effective, dCas12a-activator (dCas12a-miniVPR) for all GFP-targeting experiments. Second, both the Liu et al. and Zetsche et al. studies used CRISPR editing rather than CRISPRa. We speculate that this might explain their relatively high indel frequency: Only a single cleavage event needs to take place for an indel to occur, whereas gene activation presumably requires the dCas12a-activator to be present on the promoter for extended periods of time. Thus, any inefficiency in DNA binding caused by the separator remaining attached to the spacer might disfavor CRISPRa activity more than CRISPR-editing activity. We have added these considerations to the Discussion and referenced the suggested papers (Line 376).
Appendix Figure 1: Percentage of GFP+ cells without or with a full-length separator using dCas12a-VPR (full length) gene activation.
Relating to the above point, the vast majority of the results relied on a single guide sequence targeting GFP. While the seven-guide CRISPR array did involve other sequences, only the same GFP targeting guide yielded strong gene activation. Therefore, the generalizability of the conclusions remains unclear.
We have now performed several experiments that address the generalizability of our conclusions:
First, we now include data demonstrating that the beneficial effect of adding a synSeparator is not limited to the AAAT sequence derived from the Lachnospiraceae bacterium separator. We now include three other 4-nt, AT-rich synSeparators derived from Acidaminococcus s. (TTTT), Moraxella b. (TTTA) and Prevotella d. (ATTT) (Fig. 3I). All these synSeparators rescued the poor GFP activation caused by an upstream spacer with high GC content, though not equally effectively. The quantitative difference between the synSeparators could either be due to the intrinsic “insulation capacity” of these sequences, or the way they interact with the Lb-Cas12a protein, or to sequence-specific interactions with this particular CRISPR array. We discuss these possibilities in the Discussion (Line 437).
Second, we now include data demonstrating that nuclease-deactivated, enhanced-Cas12a from Acidaminococcus species (enAsdCas12a; Kleinstiver et al., 2019) is also sensitive to the effects of high-GC spacers (Fig. 3J). This poor performance was largely rescued by including a TTTT synSeparator derived from the natural AsCas12a separator.
Furthermore, we have now included a paragraph in the Discussion where we speculate on why the effect of adding the synSeparator was more modest for the endogenous genes than for GFP: 1) Our GFP-expressing cell line has multiple GFP insertions in its genome, and each copy has seven protospacers in its promoter. This may amplify the effect of the synSeparator. 2) The gRNAs used for endogenous activation were taken from the literature or had been pre-tested by us. These guides had thus already proven to be successful and might not be particularly disruptive (e.g., they were not selected by us for having high GC content). Therefore, researchers might experience the greatest benefit from the synSeparator with newly designed spacers that have not already proven to be effective even without the synSeparator.
Reviewer #3 (Public Review):
Magnusson et al., do an excellent job of defining how the repeated separator sequence of Wild Type Cas12a CRISPR arrays impacts the relative efficacy of downstream crRNAs in engineered delivery systems. High-GC content, particularly near the 3' end of the separator sequence appears to be critically important for the processing of a downstream crRNA. The authors demonstrated naturally occurring separators from 3 Cas12a species also display reduced GC content. The authors use this important new information to construct a synthetic small separator DNA sequence which can enhance CRISPR/Cas12a-based gene regulation in human cells. The manuscript will be a great resource for the synthetic biology field as it shows an optimization to a tool that will enable improved multi-gene transcriptional regulation.
Strengths:
- The authors do an excellent job in citing appropriate references to support the rationale behind their hypotheses.
- The experiments and results support the authors' conclusions (e.g., showing the relationship between secondary structure and GC content in the spacers).
- The controls used for the experiments were appropriate (e.g., using full-length natural separator vs single G or 1 to 4 A/T nucleotides as synthetic separators).
- The manuscript does a great job assessing several reasons why the synthetic separator might work in the discussion section, cites the relevant literature on what has been done and restates their results to argument in favor or against these reasons.
- This paper will be very useful for research groups in the genome editing and synthetic biology fields. The data presented (specially the data concerning the activation of several genes) can be used as a comparison point for other labs comparing different CRISPR-based transcriptional regulators and the spacers used for targeting.
- This paper also provides optimization to a tool that will be useful for regulating several endogenous genes at once in human cells thus helping researchers studying pathways or other functional relationships between several genes.
Opportunities for Improvement:
- The authors have performed all the experiments using LbCas12a as a model and have conclusively proven that the synSeparator enhances the performance of Cas12a based gene activation. Is this phenomenon will be same for other Cas12a proteins (such as AsCas12a)? The authors should perform some experiments to test the universality of the concept. Ideally, this would be done in HEK293T cells and one other human cell type.
We thank the reviewer for these suggestions. We have now addressed the generalizability of our findings with several new experiments. First, we now include data demonstrating that nuclease-deactivated, enhanced Cas12a from Acidaminococcus species (denAsCas12a; Kleinstiver et al., 2019) is also sensitive to the effects of high-GC spacers (Fig. 3J). This poor performance was largely rescued by including a TTTT synSeparator derived from the natural AsCas12a separator.
Second, we now include data demonstrating that the beneficial effect of adding a synSeparator is not limited to the AAAT sequence derived from the Lachnospiraceae b. separator. We now include three other 4-nt, AT-rich synSeparators derived from Acidaminococcus s. (TTTT), Moraxella b. (TTTA) and Prevotella d. (ATTT) (Fig. 3I). All these synSeparators rescued the poor GFP activation caused by an upstream spacer with high GC content, though not equally effectively. The quantitative difference between the synSeparators could either be due to the intrinsic “insulation capacity” of these sequences, or the way they interact with the Lb-Cas12a protein, or to sequence-specific interactions with this particular CRISPR array. We discuss these possibilities in the Discussion.
Third, as described above, we have now performed an in vitro Cas12a cleavage assay and present the data in a new figure (Fig. 4). We found that a CRISPR array containing a 70%-GC dummy spacer was processed less efficiently than an array containing a 30%-GC spacer, but that addition of a synSeparator could to a large extent rescue this processing defect (Fig. 4E). The fact that this result was observed even in a cell-free in vitro setting demonstrates that it is a general feature of Cas12a CRISPR arrays that is likely to work the same way in many cell types rather than being specific to HEK293T cells.
Fourth, we attempted to investigate the effect of the synSeparator in different cell types. However, either due to poor transfection efficiency or poor expression of the Cas12a activator construct, CRISPRa activity was consistently poor in these cell types, both with and without the synSeparator (e.g., we did not visually observe fluorescence from the mCherry gene fused to the dCas12a activator, which we always see in HEK293T cells). Because of the low general efficiency of CRISPRa, it was not possible to evaluate the performance of the synSeparator. Many cell types are difficult to transfect and dCas12a-VPR-mCherry is a big construct (>6 kb). To our knowledge, there have not been many reports using dCas12a-VPR in cell types other than HEK293T. While we think that it will be important to optimize CRISPRa in many cell types (e.g., by optimizing transfection conditions, Cas12a variants, promoters, expression vectors, etc.), the focus of our study has been to show the separator’s mechanism and general function; we believe that optimizing general CRISPRa for different cell types is beyond the scope of this paper. We acknowledge that this is a limitation of our study and we have added a paragraph about this in the Discussion (line 355). We nevertheless hypothesize that the negative influence of high-GC spacers and the insulating effect of synSeparators are generalizable across cell types. That is because we could observe improved array processing with the synSeparator even in the cell-free context of an in vitro expression system, as described above (Fig. 4). This suggests that the sensitivity to spacer GC content is determined only by the interaction between Cas12a and the array, rather than being dependent on a particular cellular context.
-

Reviewer #3 (Public Review):
Magnusson et al., do an excellent job of defining how the repeated separator sequence of Wild Type Cas12a CRISPR arrays impacts the relative efficacy of downstream crRNAs in engineered delivery systems. High-GC content, particularly near the 3' end of the separator sequence appears to be critically important for the processing of a downstream crRNA. The authors demonstrated naturally occurring separators from 3 Cas12a species also display reduced GC content. The authors use this important new information to construct a synthetic small separator DNA sequence which can enhance CRISPR/Cas12a-based gene regulation in human cells. The manuscript will be a great resource for the synthetic biology field as it shows an optimization to a tool that will enable improved multi-gene transcriptional regulation.
Strengths:
Reviewer #3 (Public Review):
Magnusson et al., do an excellent job of defining how the repeated separator sequence of Wild Type Cas12a CRISPR arrays impacts the relative efficacy of downstream crRNAs in engineered delivery systems. High-GC content, particularly near the 3' end of the separator sequence appears to be critically important for the processing of a downstream crRNA. The authors demonstrated naturally occurring separators from 3 Cas12a species also display reduced GC content. The authors use this important new information to construct a synthetic small separator DNA sequence which can enhance CRISPR/Cas12a-based gene regulation in human cells. The manuscript will be a great resource for the synthetic biology field as it shows an optimization to a tool that will enable improved multi-gene transcriptional regulation.
Strengths:
- The authors do an excellent job in citing appropriate references to support the rationale behind their hypotheses.
- The experiments and results support the authors' conclusions (e.g., showing the relationship between secondary structure and GC content in the spacers).
- The controls used for the experiments were appropriate (e.g., using full-length natural separator vs single G or 1 to 4 A/T nucleotides as synthetic separators).
- The manuscript does a great job assessing several reasons why the synthetic separator might work in the discussion section, cites the relevant literature on what has been done and restates their results to argument in favor or against these reasons.
- This paper will be very useful for research groups in the genome editing and synthetic biology fields. The data presented (specially the data concerning the activation of several genes) can be used as a comparison point for other labs comparing different CRISPR-based transcriptional regulators and the spacers used for targeting.
- This paper also provides optimization to a tool that will be useful for regulating several endogenous genes at once in human cells thus helping researchers studying pathways or other functional relationships between several genes.
Opportunities for Improvement:
- The authors have performed all the experiments using LbCas12a as a model and have conclusively proven that the synSeparator enhances the performance of Cas12a based gene activation. Is this phenomenon will be same for other Cas12a proteins (such as AsCas12a)? The authors should perform some experiments to test the universality of the concept. Ideally, this would be done in HEK293T cells and one other human cell type.
-

Reviewer #2 (Public Review):
Type V CRISPR-Cas systems are used in a variety of biotechnology applications, which rely on the association of a Cas12a-CRISPR RNA complex association with a complementary target DNA sequence. One advantage of the Cas12a system over other CRISPR-Cas systems is the ability to multiplex by expressing multiple CRISPR RNAs in an array, with the individual RNAs processed from a longer transcript by Cas12a. Magnusson et al. show that the activity of CRISPR RNAs in this system is enhanced by including a short, A/T-rich sequence between each encoded CRISPR RNA. The authors propose that these separator sequences reduce the potential for secondary structure, thereby promoting RNA processing. This is an exciting idea, with obvious applications wherever Cas12a is used. However, while the presented data are consistent …
Reviewer #2 (Public Review):
Type V CRISPR-Cas systems are used in a variety of biotechnology applications, which rely on the association of a Cas12a-CRISPR RNA complex association with a complementary target DNA sequence. One advantage of the Cas12a system over other CRISPR-Cas systems is the ability to multiplex by expressing multiple CRISPR RNAs in an array, with the individual RNAs processed from a longer transcript by Cas12a. Magnusson et al. show that the activity of CRISPR RNAs in this system is enhanced by including a short, A/T-rich sequence between each encoded CRISPR RNA. The authors propose that these separator sequences reduce the potential for secondary structure, thereby promoting RNA processing. This is an exciting idea, with obvious applications wherever Cas12a is used. However, while the presented data are consistent with the model, I think the conclusions are too preliminary, and require (i) a more targeted assessment of the importance of RNA secondary structure for RNA processing, (ii) direct measurement of RNA processing, and (iii) a more extensive assessment of the effect of adding spacer sequences to CRISPR arrays in a functional assay.
-

Reviewer #1 (Public Review):
The authors interrogated an underexplored feature of CRISPR arrays to enhance multiplexed genome engineering with the CRISPR nuclease Cas12a. Multiplexing represents one of the many desirable features of CRISPR technologies, and use of highly compact CRISPR arrays from CRISPR-Cas systems allows targeting of many sites at one time. Recent work has shown though that the composition of the array can have a major impact on the performance of individual guide RNAs encoded within the array, providing ample opportunities for further improvements. In this manuscript, the authors found that the region within the repeat lost through processing, what they term the separator, can have a major impact on targeting performance. The effect was specifically tied to upstream guide sequences with high GC content. Introducing …
Reviewer #1 (Public Review):
The authors interrogated an underexplored feature of CRISPR arrays to enhance multiplexed genome engineering with the CRISPR nuclease Cas12a. Multiplexing represents one of the many desirable features of CRISPR technologies, and use of highly compact CRISPR arrays from CRISPR-Cas systems allows targeting of many sites at one time. Recent work has shown though that the composition of the array can have a major impact on the performance of individual guide RNAs encoded within the array, providing ample opportunities for further improvements. In this manuscript, the authors found that the region within the repeat lost through processing, what they term the separator, can have a major impact on targeting performance. The effect was specifically tied to upstream guide sequences with high GC content. Introducing synthetic separator sequences shorter than their natural counterparts but exhibiting similarly low GC content boosted targeted activation of a reporter in human cells. Applying one synthetic separator to a seven-guide array targeting chromosomal genes led to consistent though more modest targeted activation. These findings introduce a distinct design consideration for CRISPR arrays that can further enhance the efficacy of multiplexed applications. The findings also suggest a selective pressure potentially influencing the repeat sequence in natural CRISPR arrays.
Strengths:
The portion of the repeat discarded through processing normally has been included or discarded when generating a CRISPR-Cas12a array. The authors clearly show that something in between-namely using a short version with a similarly low GC content-can enhance targeting over the truncated version. A coinciding surprising result was that the natural separator completely eliminated any measurable activation, necessitating the synthetic separator.
The manuscript provides a clear progression from identifying a feature of the upstream sequences impacting targeting to gaining insights from natural CRISPR-Cas12a systems to applying the insights to enhance array performance.
With further support, the use of synthetic separators could be widely adopted across the many applications of CRISPR-Cas12a arrays.
Weaknesses:
The terminology used to describe the different parts of the CRISPR array could better align with those in the CRISPR biology field. For one, crRNAs (abbreviated from CRISPR RNAs) should reflect the final processed form of the guide RNA, whereas guide RNAs (gRNAs) captures both pre-processed and post-processed forms. Also, "spacers" should reflect the natural spacers acquired by the CRISPR-Cas system, whereas "guides" better capture the final sequence in the gRNA used for DNA target recognition.
A running argument of the work is that the separator specifically evolved to buffer adjacent crRNAs. However, this argument overlooks two key aspects of natural CRISPR arrays. First, the spacer (~30 nts) is normally much longer than the guide used in this work (20 nts), already providing the buffer described by the authors. This spacer also undergoes trimming to form the mature crRNA. Second, the repeat length is normally fixed as a consequence of the mechanisms of spacer acquisition. At most, the beginning of each repeat sequence may have evolved to reduce folding interactions without changing the repeat length, although some of these repeats are predicted to fold into small hairpins.
Prior literature has highlighted the importance of a folded hairpin with an upstream pseudoknot within the repeat (Yamano Cell 2016), where disrupting this structure compromises DNA targeting by Cas12a (Liao Nat Commun 2019, Creutzburg NAR 2020). This structure is likely central to the authors' findings and needs to be incorporated into the analyses.
Many claims could better reflect the cited literature. For instance, Creutzburg et al. showed that adding secondary structures to the guide to promote folding of the repeat hairpin enhanced rather than interfered with targeting. Liu et al. NAR 2019 further showed that the pre-processed repeat actually enhanced rather than reduced performance compared to the processed repeat. Finally, the complete loss of targeting with the unprocessed repeat appears represent an extreme example given multiple studies that showed effective targeting with this repeat (e.g. Liu NAR 2019, Zetsche Nat Biotechnol 2016).
Relating to the above point, the vast majority of the results relied on a single guide sequence targeting GFP. While the seven-guide CRISPR array did involve other sequences, only the same GFP targeting guide yielded strong gene activation. Therefore, the generalizability of the conclusions remains unclear.
-

Evaluation Summary:
This manuscript is of broad interest to those performing multiplexed genome engineering and related applications with CRISPR Cas12a technologies. While the proposed use of synSeparators is promising, the paper would benefit from further investigation of the mechanism by which synSeparators function to promote Cas12a activity. Additional data would be required to support the current conclusions regarding the generalizability of the findings.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
-