Translation Inhibitory Elements from Hox a3 and a11 mRNAs use uORFs for translation inhibition
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Non-canonical pathways for regulating protein synthesis in animals serve important roles for controlling gene expression in critical developmental pathways. Homeobox (Hox) genes encode many mRNAs regulated at the level of translation. A general feature for many of these mRNAs has been the proposal they are regulated by Internal Ribosome Entry Sites and possess sequences in the 5'-untranslated regions of the mRNA that prevent canonical cap-dependent translation, termed "translation inhibitory elements". Here, the authors focus on two Hox mRNAs and find they use entirely different means to achieve the same end of repressing cap-dependent translation. Overall, the experiments support the major conclusions drawn by the authors, and nail down mechanisms that have been left unresolved since the Hox mRNAs were first discovered to be regulated at the level of translation.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
During embryogenesis, Hox mRNA translation is tightly regulated by a sophisticated molecular mechanism that combines two RNA regulons located in their 5’UTR. First, an Internal Ribosome Entry Site (IRES) enables cap-independent translation. The second regulon is a Translation Inhibitory Element or TIE, which ensures concomitant cap-dependent translation inhibition. In this study, we deciphered the molecular mechanisms of Hox a3 and a11 TIE elements. Both TIEs possess an upstream Open Reading Frame (uORF) that is critical to inhibit cap-dependent translation. However, the molecular mechanisms used are different. In TIE a3, we identify a uORF which inhibits cap-dependent translation and we show the requirement of the non-canonical initiation factor eIF2D for this process. The mode of action of TIE a11 is different, it also contains a uORF but it is a minimal uORF formed by an uAUG followed immediately by a stop codon, namely a ‘start-stop’. The a11 ‘start-stop’ sequence is located upstream of a highly stable stem loop structure which stalls the 80S ribosome and thereby inhibits cap-dependent translation of Hox a11 main ORF.
Article activity feed
-

Author Response:
Reviewer #2:
Non-canonical pathways for regulating protein synthesis serve important roles for controlling gene expression in critical developmental pathways. Homeobox (Hox) genes encode many mRNAs regulated at the level of translation. A general feature for many of these mRNAs has been the proposal they are regulated by Internal Ribosome Entry Sites (IRESs) and possess sequences in the 5'-untranslated regions (5'-UTR) of the mRNA that prevent canonical cap-dependent translation, termed "translation inhibitory elements" or TIEs. However, the mechanisms by which these Hox mRNAs are regulated remain unclear. Here, the authors focus on two Hox mRNAs, Hox a3 and Hox a11, and find they use entirely different means to achieve the same end of repressing cap-dependent translation. Hox a3 uses the non-canonical translation …
Author Response:
Reviewer #2:
Non-canonical pathways for regulating protein synthesis serve important roles for controlling gene expression in critical developmental pathways. Homeobox (Hox) genes encode many mRNAs regulated at the level of translation. A general feature for many of these mRNAs has been the proposal they are regulated by Internal Ribosome Entry Sites (IRESs) and possess sequences in the 5'-untranslated regions (5'-UTR) of the mRNA that prevent canonical cap-dependent translation, termed "translation inhibitory elements" or TIEs. However, the mechanisms by which these Hox mRNAs are regulated remain unclear. Here, the authors focus on two Hox mRNAs, Hox a3 and Hox a11, and find they use entirely different means to achieve the same end of repressing cap-dependent translation. Hox a3 uses the non-canonical translation initiation factor eIF2D and an upstream open reading fram (uORF), whereas a11 uses a "start-stop" uORF followed by a thermodynamically stable stem-loop to inhibit translation. Overall, the experiments support the major conclusions drawn by the authors, and nail down mechanisms that have been left unresolved since the Hox mRNAs were first discovered to be regulated at the level of translation. These results will be of wide interest to the translation and developmental biology fields.
Some issues the authors should consider:
- The mapping of the TIE boundaries are in general well-supported by the luciferase reporter experiments. However, there seems to be a disconnect in the luciferase values in Fig. 1B compared to the western blots in Supplementary Fig. 1D, however. For example, in the a3 case the 106 and 113 bands don't seem to correspond to levels consistent with the luciferase activity. For a11, the 153 band is not consistent with the luciferase activity. Also, the gels at the bottom are confusing. Should 74 in the left gel be 77? It would help to have a clearer explanation in the figure legend.
The reviewer is right, supplementary figure 1D is misleading. We have clarified the data with a new supplementary figure 1D. The gels presented in this figure are not western blots, they are SDS-page analysis of translated product (i.e. Renilla luciferase protein) in the presence of 35S-Methionin. Since the function of TIE elements was measured in comparison with reporters that do not contain any TIE element, we loaded on each gel a reference (lanes w/o TIE) for quantification purposes. Since the exposure time of distinct gels was variable, one should not compare the intensities in between gels. We added the quantification of the gel intensity related to the reference construct (w/o TIE). We agree with the reviewer that the two gels at the bottom are not informative, we removed them from the new supplemental figure 1D.
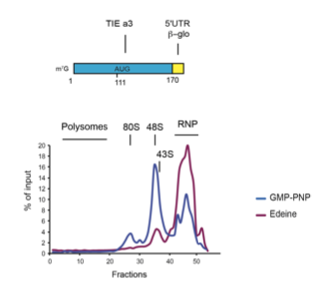
- The results in the various sucrose gradients are not entirely convincing as presented. In all these cases, the experiment would benefit from the use of high-salt conditions (See Lodish and Rose, 1977, JBC 252, 1181-ff) in the gradient to remove background 80S not engaged with mRNAs. For the +cycloheximide sample in Fig. 8, this looks more like a "half-mer" between a monosome and disome, rather than a standard polysome.
We do not agree with the point raised by the reviewer on sucrose gradients. Obviously this is due to a misunderstanding of the conducted experiments. We would like to remind that the plots shown in the manuscript represent the percentage of mRNA transcripts labelled with a radioactive cap that were introduced in cell-free translation extracts. Therefore, since we monitor only radioactivity, the sole radioactive mRNA transcripts tested in these experiments are observed, consequently there is no background 80S that are not engaged with mRNAs. Such background 80S are visible on the OD profile shown now in a novel supplementary figure S6. However, non-engaged 80S are not radioactive and mRNAs that are not engaged in the 80S are found in the RNP fraction. The absence of radioactive background 80S is further corroborated by the use of edeine that prevents the codon-anticodon interaction (see data below).
When we setup our experimental strategy, we first used edeine to validate our protocol, in this case no radioactive 80S is observed confirming that no background 80S is present in our assays. In conclusion, peaks at the level of 80S can only be radioactive mRNA engaged in an 80S. We have extended the figure legend to clarify the conducted experiments.
Concerning Fig 8, we agree that this experiment is not conclusive and propose to remove it as mentioned in response to a comment from reviewer #1.
- In Fig. 7, it would be helpful to see the absolute level of translation from the reporters, as it is not clear what the baseline level of translation is in the knockdown cell lines. It's hard to judge the eIF4E knockdown case in particular without this information. Also in panel B, the GGCCC147 cell line is missing.
As previously mentioned, we agree that Fig 7 is misleading and we have completely remodelled the figure in the revised manuscript. See also point 5 from reviewer #1. Because the GGCCC147 mutation had no effect in RRL, we decided not to test it in HEK cells and focused on the GGCC107 that has a significant effect both in RRL and in HEK cells.
- From the MS experiments in Fig. 6 and Supplementary Fig. 6, the authors focus on eIF2D, which makes sense. But they don't comment on two other highly suggestive hits in the a3 vs. beta-globin and a3 vs. a11 comparisons. These are eIF5B and HBS1L. Both are highly suggestive of what might be going in with the eIF2D-dependent translation mechanism. They don't show up in the GMP-PNP samples in Supplementary Fig. 6, which is interesting and would deserve a comment.
We are grateful for this very interesting comment. As suggested, we have inserted a comment related to HBS1L and eIF5B in the discussion of the manuscript.
-

Reviewer #3 (Public Review):
Alghoul et. al are attempting to decipher the molecular mechanisms of Hox a3 and a11 TIE elements to inhibit cap-dependent translation. It is known that both TIEs possess an upstream Open Reading Frame (uORF) that is critical. Here they seek to understand the exact molecular mechanism used for inhibition. They were able to show that both a3 and a11 are regulated by different mechanisms to ensure the inhibition of ca-dependent translation. They found that the translation inhibitory mechanism mediated by TIE a3 requires the presence of the translation initiation factor eIF2D. However, the mechanism mediated by TIE a11 contains three elements that enable a highly efficient inhibition of cap-dependent translation, these are: an upstream start codon (uAUG), followed by a stop codon, and a long stable hairpin. …
Reviewer #3 (Public Review):
Alghoul et. al are attempting to decipher the molecular mechanisms of Hox a3 and a11 TIE elements to inhibit cap-dependent translation. It is known that both TIEs possess an upstream Open Reading Frame (uORF) that is critical. Here they seek to understand the exact molecular mechanism used for inhibition. They were able to show that both a3 and a11 are regulated by different mechanisms to ensure the inhibition of ca-dependent translation. They found that the translation inhibitory mechanism mediated by TIE a3 requires the presence of the translation initiation factor eIF2D. However, the mechanism mediated by TIE a11 contains three elements that enable a highly efficient inhibition of cap-dependent translation, these are: an upstream start codon (uAUG), followed by a stop codon, and a long stable hairpin. These findings show that these TIE elements of Hox mRNAs enable regulatory control between canonical translation and non-canonical translation Internal Ribosome Entry Site (IRES) translation.
The authors use a vast amount of different sophisticated techniques to prove the molecular mechanism of inhibition conferred by the TIE elements. They start by cloning the regions upstream of the beta-globin 5'UTR in a RLuc vector and sequentially deleting regions to identify the region that confers inhibition. By using chemical modification probing, they confirm RNA secondary structure and identify regions of interest that might be responsible for inhibition. Then they focus of each element separately. In TIE a3, they identified an uORF that requires eIF2D for this process. They used MS analysis to identify the binding partners of the two elements and they further confirmed by silencing eIF2D that the inhibition doesn't occur in its absence. They further corroborated this finding by mutating an A-rich sequence found upstream of the uAUG that determines specificity of eIF2D binding. In the a11 case, they use toe-printing and mutagenesis to determine that a 'start-stop' sequence is located upstream of a highly stable stem loop structure which stalls the 80S ribosome and thereby inhibits cap-dependent translation of Hox a11 main ORF.
-

Reviewer #2 (Public Review):
Non-canonical pathways for regulating protein synthesis serve important roles for controlling gene expression in critical developmental pathways. Homeobox (Hox) genes encode many mRNAs regulated at the level of translation. A general feature for many of these mRNAs has been the proposal they are regulated by Internal Ribosome Entry Sites (IRESs) and possess sequences in the 5'-untranslated regions (5'-UTR) of the mRNA that prevent canonical cap-dependent translation, termed "translation inhibitory elements" or TIEs. However, the mechanisms by which these Hox mRNAs are regulated remain unclear. Here, the authors focus on two Hox mRNAs, Hox a3 and Hox a11, and find they use entirely different means to achieve the same end of repressing cap-dependent translation. Hox a3 uses the non-canonical translation …
Reviewer #2 (Public Review):
Non-canonical pathways for regulating protein synthesis serve important roles for controlling gene expression in critical developmental pathways. Homeobox (Hox) genes encode many mRNAs regulated at the level of translation. A general feature for many of these mRNAs has been the proposal they are regulated by Internal Ribosome Entry Sites (IRESs) and possess sequences in the 5'-untranslated regions (5'-UTR) of the mRNA that prevent canonical cap-dependent translation, termed "translation inhibitory elements" or TIEs. However, the mechanisms by which these Hox mRNAs are regulated remain unclear. Here, the authors focus on two Hox mRNAs, Hox a3 and Hox a11, and find they use entirely different means to achieve the same end of repressing cap-dependent translation. Hox a3 uses the non-canonical translation initiation factor eIF2D and an upstream open reading fram (uORF), whereas a11 uses a "start-stop" uORF followed by a thermodynamically stable stem-loop to inhibit translation. Overall, the experiments support the major conclusions drawn by the authors, and nail down mechanisms that have been left unresolved since the Hox mRNAs were first discovered to be regulated at the level of translation. These results will be of wide interest to the translation and developmental biology fields.
Some issues the authors should consider:
The mapping of the TIE boundaries are in general well-supported by the luciferase reporter experiments. However, there seems to be a disconnect in the luciferase values in Fig. 1B compared to the western blots in Supplementary Fig. 1D, however. For example, in the a3 case the 106 and 113 bands don't seem to correspond to levels consistent with the luciferase activity. For a11, the 153 band is not consistent with the luciferase activity. Also, the gels at the bottom are confusing. Should 74 in the left gel be 77? It would help to have a clearer explanation in the figure legend.
The results in the various sucrose gradients are not entirely convincing as presented. In all these cases, the experiment would benefit from the use of high-salt conditions (See Lodish and Rose, 1977, JBC 252, 1181-ff) in the gradient to remove background 80S not engaged with mRNAs. For the +cycloheximide sample in Fig. 8, this looks more like a "half-mer" between a monosome and disome, rather than a standard polysome.
In Fig. 7, it would be helpful to see the absolute level of translation from the reporters, as it is not clear what the baseline level of translation is in the knockdown cell lines. It's hard to judge the eIF4E knockdown case in particular without this information. Also in panel B, the GGCCC147 cell line is missing.
From the MS experiments in Fig. 6 and Supplementary Fig. 6, the authors focus on eIF2D, which makes sense. But they don't comment on two other highly suggestive hits in the a3 vs. beta-globin and a3 vs. a11 comparisons. These are eIF5B and HBS1L. Both are highly suggestive of what might be going in with the eIF2D-dependent translation mechanism. They don't show up in the GMP-PNP samples in Supplementary Fig. 6, which is interesting and would deserve a comment.
-

Reviewer #1 (Public Review):
In this manuscript entitled "Translation inhibitory elements from Hoxa3 and a11 mRNAs use uORFs for translation inhibition", the authors undertake a series of in vitro translation and in cell experiments to characterize the inhibitory features of previously documented Hoxa11 and Hox3 translation inhibitory elements (TIEs). The presence of TIEs within a subset of Hox mRNAs are thought to mediate repression of cap-dependent translation, enabling downstream IRES-mediated initiation to proceed. In sum, the authors report: (i) the presence of an upstream uORF in the Hoxa3 TIE sequence that dampens translation from the downstream ORF and (ii) the presence of a stem-loop structure that appears to block ribosome migration and results in inhibition of the downstream ORF (even thought the 5' UTR of a11 also has 2 …
Reviewer #1 (Public Review):
In this manuscript entitled "Translation inhibitory elements from Hoxa3 and a11 mRNAs use uORFs for translation inhibition", the authors undertake a series of in vitro translation and in cell experiments to characterize the inhibitory features of previously documented Hoxa11 and Hox3 translation inhibitory elements (TIEs). The presence of TIEs within a subset of Hox mRNAs are thought to mediate repression of cap-dependent translation, enabling downstream IRES-mediated initiation to proceed. In sum, the authors report: (i) the presence of an upstream uORF in the Hoxa3 TIE sequence that dampens translation from the downstream ORF and (ii) the presence of a stem-loop structure that appears to block ribosome migration and results in inhibition of the downstream ORF (even thought the 5' UTR of a11 also has 2 uAUGs - these do not appear to play a determining role in a11 TIE activity).
Major Strengths: This study is comprehensive and thorough. The manuscript is well written.
Weaknesses: Some of the experiments lacked internal controls making interpretation of the results preliminary in nature.
For the most part, the authors have defined the inhibitory features of the Hox a3 and a11 Translation Inhibitory Element. The work was placed in appropriate context (Introduction). The work further supports the known concept that uAUGs and 5' UTR secondary structure is detrimental to eukaryotic translation inhibition.
-

Evaluation Summary:
Non-canonical pathways for regulating protein synthesis in animals serve important roles for controlling gene expression in critical developmental pathways. Homeobox (Hox) genes encode many mRNAs regulated at the level of translation. A general feature for many of these mRNAs has been the proposal they are regulated by Internal Ribosome Entry Sites and possess sequences in the 5'-untranslated regions of the mRNA that prevent canonical cap-dependent translation, termed "translation inhibitory elements". Here, the authors focus on two Hox mRNAs and find they use entirely different means to achieve the same end of repressing cap-dependent translation. Overall, the experiments support the major conclusions drawn by the authors, and nail down mechanisms that have been left unresolved since the Hox mRNAs were first …
Evaluation Summary:
Non-canonical pathways for regulating protein synthesis in animals serve important roles for controlling gene expression in critical developmental pathways. Homeobox (Hox) genes encode many mRNAs regulated at the level of translation. A general feature for many of these mRNAs has been the proposal they are regulated by Internal Ribosome Entry Sites and possess sequences in the 5'-untranslated regions of the mRNA that prevent canonical cap-dependent translation, termed "translation inhibitory elements". Here, the authors focus on two Hox mRNAs and find they use entirely different means to achieve the same end of repressing cap-dependent translation. Overall, the experiments support the major conclusions drawn by the authors, and nail down mechanisms that have been left unresolved since the Hox mRNAs were first discovered to be regulated at the level of translation.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-