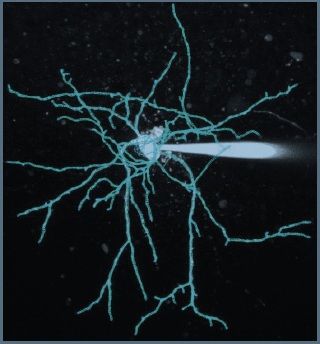
Developmental emergence of two-stage nonlinear synaptic integration in cerebellar interneurons
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Using a range of cutting-edge techniques, the authors of this manuscript explore the functional consequences of developmental changes in dendritic morphology and synapse distribution in a GABAergic interneuron. The experiments are carefully performed and represent a thorough investigation of the structural and functional changes in synaptic inputs and postsynaptic dendritic branching patterns, an important topic for both developmental neurobiologists and synaptic physiologists. The data support most conclusions, but alternative interpretations remain possible and should be further considered.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Synaptic transmission, connectivity, and dendritic morphology mature in parallel during brain development and are often disrupted in neurodevelopmental disorders. Yet how these changes influence the neuronal computations necessary for normal brain function are not well understood. To identify cellular mechanisms underlying the maturation of synaptic integration in interneurons, we combined patch-clamp recordings of excitatory inputs in mouse cerebellar stellate cells (SCs), three-dimensional reconstruction of SC morphology with excitatory synapse location, and biophysical modeling. We found that postnatal maturation of postsynaptic strength was homogeneously reduced along the somatodendritic axis, but dendritic integration was always sublinear. However, dendritic branching increased without changes in synapse density, leading to a substantial gain in distal inputs. Thus, changes in synapse distribution, rather than dendrite cable properties, are the dominant mechanism underlying the maturation of neuronal computation. These mechanisms favor the emergence of a spatially compartmentalized two-stage integration model promoting location-dependent integration within dendritic subunits.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
Here the authors use a variety of sophisticated approaches to assess the contribution of synaptic parameters to dendritic integration across neuronal maturation. They provide high-quality data identifying cellular parameters that underlie differences in AMPAR-mediated synaptic currents measured between adolescent and adult cerebellar stellate cells, and conclude that differences are attributed to an increase in the complexity of the dendritic arbor. This conclusion relies primarily on the ability of a previously described model for adult stellate cells to recapitulate the age-dependent changes in EPSCs by a change in dendritic branching with no change in synapse density. These rigorous results have implications for understanding how changing structure during neuronal development affects …
Author Response:
Reviewer #1 (Public Review):
Here the authors use a variety of sophisticated approaches to assess the contribution of synaptic parameters to dendritic integration across neuronal maturation. They provide high-quality data identifying cellular parameters that underlie differences in AMPAR-mediated synaptic currents measured between adolescent and adult cerebellar stellate cells, and conclude that differences are attributed to an increase in the complexity of the dendritic arbor. This conclusion relies primarily on the ability of a previously described model for adult stellate cells to recapitulate the age-dependent changes in EPSCs by a change in dendritic branching with no change in synapse density. These rigorous results have implications for understanding how changing structure during neuronal development affects integration of AMPR-mediated synaptic responses.
The data showing that younger SCs have smaller dendritic arbors but similar synapse density is well-documented and provides compelling evidence that these structural changes affect dendritic integration. But the main conclusion also relies on the assumption that the biophysical model built for adult SCs applies to adolescent SCs, and there are additional relevant variables related to synaptic function that have not been fully assessed. Thus, the main conclusions would be strengthened and broadened by additional experimental validation.
We thank the reviewer for the positive assessment of the quality and importance of our manuscript. Below we address the reviewer’s comments directly but would like to stress that the goal of the manuscript was to understand the cellular mechanisms underlying developmental slowing of mEPSCs in SCs and the consequent implication for developmental changes in dendritic integration, which have rarely been examined to date, and not to establish a detailed biophysical model of cerebellar SCs. The latter would require dual-electrode recordings (one on 0.5 um dendrites), detailed description of the expression, dendritic localization of the gap junction protein connexin 36 (as done in Szoboszlay neuron 2016), and a detailed description prameter variability across the SC population (e.g. variations in AMPAR content at synapses, Rm, and dendritic morphology). Such experiments are well beyond the scope of the manuscript. Here we use biophysical simulations to support conclusions derived from specific experiments, more as a proof of principle rather than a strict quantitative prediction.
Nevertheless, we would like to clarify our selection of parameters for the biophysical models for immature and adult SCs. We did not simply “assume” that the biophysical models were the same at the two developmental stages. We either used evidence from the literature or our own measured parameters to establish an immature SC model. As compared to adult SCs, we found that immature SCs had 1) an identical membrane time constant, 2) an only slightly larger dendrite diameter, 3) decreased dendritic branching and maximum lengths, 4) a comparable synapse density, and 5) a homogeneous synapse distribution. Taken together, we concluded that increased dendritic branching during SC maturation resulted in a larger fraction of synapses at longer electrotonic distances in adult SCs. These experimental findings were incorporated into two distinct biophysical models representing immature and adult SCs. Evidence from the literature suggests that voltage-gated channels expression is not altered between the two developmental stages studied here. Therefore, like the adult SC model, we considered only the passive membrane properties and the dendritic morphology. The simulation results supported our conclusion that the increased apparent dendritic filtering of mEPSCs resulted from a change in the distribution of synapse distance to the soma rather than cable properties. Some of the measured parameters (e.g., membrane time constant) were not clearly stated manuscript, which we have corrected in the revised manuscript.
We are not sure what the reviewer meant by suggesting that we did not examine “other relevant variables related to synaptic function.” Later, the reviewer refers to alterations in AMPAR subunit composition or changes in cleft glutamate concentration (low-affinity AMPAR antagonist experiments). We performed experiments to directly examine both possible contributions by comparing qEPSC kinetics and performing low-affinity antagonist experiments, respectively, but we found that neither mechanism could account for the developmental slowing of mEPSCs. We, therefore, did not explore further possible developmental changes AMPAR subunits. See below for a more specific response and above for newly added text.
While many exciting questions could be examined in the future, we do not think the present study requires additional experiments. Nevertheless, we recognize that perhaps we can improve the description of the results to justify our conclusions better (see specifics below).
Reviewer #2 (Public Review):
This manuscript investigates the cellular mechanisms underlying the maturation of synaptic integration in molecular layer interneurons in the cerebellar cortex. The authors use an impressive combination of techniques to address this question: patch-clamp recordings, 2-photon and electron microscopy, and compartmental modelling. The study builds conceptually and technically on previous work by these authors (Abrahamsson et al. 2012) and extends the principles described in that paper to investigate how developmental changes in dendritic morphology, synapse distribution and strength combine to determine the impact of synaptic inputs at the soma.
- Models are constructed to confirm the interpretation of experimental results, mostly repeating the simulations from Abrahamsson et al. (2012) using 3D reconstructed morphologies. The results are as expected from cable theory, given the (passive) model assumptions. While this confirmation is welcome and important, it is disappointing to see the opportunity missed to explore the implications of the experimental findings in greater detail. For instance, with the observed distributions of synapses, are there more segregated subunits available for computation in adult vs immature neurons?
As described in our response to reviewer 1, this manuscript intends to identify the cellular mechanisms accounting developmental slowing of mEPSCs and its implication for dendritic integration. The modeling was designed to support the most plausible explanation that increased branching resulted in more synapses at longer electrotonic distances. This finding is novel and merits more in-depth examination at a computation level in future studies.
Quantifying dendritic segregation is non-trivial due to dendritic nonlinearities and the difficulties in setting criteria for electrical “isolation” of inputs. However, because the space constant does not change with development, while both dendrite length and branching increase, it is rather logical to conclude qualitatively that the number of computational segments increases with development.
We have added the following sentence to the Discussion (line 579):
“Moreover, since the space constant does not change significantly with development and the dendritic tree complexity increases, the number of computational segments is expected to increase with development.”
How do SCs respond at different developmental stages with in vivo-like patterns of input, rather than isolated activation of synapses? Answering these sorts of questions would provide quantitative support for the conclusion that computational properties evolve with development.
While this is indeed a vital question, the in vivo patterns of synaptic activity are not known, so it is difficult to devise experiments to arrive at definitive conclusions.
- From a technical perspective, the modeling appears to be well-executed, though more methodological detail is required for it to be reproducible. The AMPA receptor model and reversal potential are unspecified, as is the procedure for fitting the kinetics to data.
We did not use an explicit channel model to generate synaptic conductances. We simply used the default multiexponential function of Neuron (single exponential rise and single exponential decay) and adjusted the parameters tauRise and tauDecay such that simulated EPSCs matched somatic quantal EPSC amplitude, rise time and τdecay (Figure 4).
We added the following text to the methods (line 708):
“The peak and kinetics of the AMPAR-mediated synaptic conductance waveforms (gsyn) were set to simulate qEPSCs that matched the amplitude and kinetics of experimental somatic quantal EPSCs and evoked EPSCs. Immature quantal gsyn had an peak amplitude of 0.00175 μS, a 10-90 % RT of 0.0748 ms and a half-width of 0.36 ms (NEURON synaptic conductance parameter Tau0 = 0.073 ms, Tau1 = 0.26 ms and Gmax = 0.004 μS) while mature quantal gsyn had an peak amplitude of 0.00133 μS, a 10-90 % RT of 0.072 ms and a half-width of 0.341 ms (NEURON synaptic conductance parameters Tau0 = 0.072 ms, Tau1 = 0.24 ms and Gmax = 0.0032 μS). For all simulations, the reversal potential was set to 0 mV and the holing membrane potential was to – 70 mV. Experimental somatic PPR for EPSCs were reproduced with a gsyn 2/ gsyn 1 of 2.25.”
Were simulations performed at resting potential, and if yes, what was the value?
The membrane potential was set at – 70 mV to match that of experimental recordings and has been updated in the Methods section.
How was the quality of the morphological reconstructions assessed? Accurate measurement of dendritic diameters is crucial to the simulations in this study, so providing additional morphometrics would be helpful for assessing the results. Will the models and morphologies be deposited in ModelDB or similar?
For the two reconstructions imported into NEURON for simulations, we manually curated the dendritic diameters to verify a matching of the estimated diameter to that of the fluorescence image using NeuroStudio, which uses a robust subpixel estimation algorithm (Rayburst diameter, Rodriguez et al. 2008). The reconstructions include all variations in diameter throughout the dendritic tree (see as a example the the result of the reconstruction on the image below for the immature SC presented in the Figure 2D). The mean diameter across the entire dendritic tree of the reconstructed immature and adult SC was 0.42 and 0.36 μm, respectively, similar to the ratio of measured diameters estimated using confocal microscopy.
We have updated the methods section to include how reconstructions were curated and analyzed (line 693).
“An immature (P16) and adult SC (P42) were patch loaded with 30 μM Alexa 594 in the pipette and imaged using 2PLSM. Both cells were reconstructed in 3D using NeuronStudio in a semiautomatic mode which uses a robust subpixel estimation algorithm (calculation of Rayburst diameter (Rodriguez et al., 2008)). We manually curated the diameters to verify that it matched the fluorescence image to faithfully account for all variations in diameter throughout the dendritic tree. The measured diameter across the entire dendritic tree of the reconstructed immature and adult SCs was 0.42 and 0.36 μm, respectively. The 16% smaller diameter in adult was similar to the 13% obtained from confocal image analysis from many SCs (see Figure 2B).”
We agree with the reviewer that accurate measurements of dendritic diameters are crucial for the simulations. We did not rely soley on the reconstructed SCs, but we also performed highresolution confocal microscopy analysis of 16 different dye-filled SCs. We examined differences in the FWHM of intensity line profiles drawn perpendicular to the dendrite between immature and adult SCs. The FWHM is a good approximation of dendritic diameter and was performed similarly to adult SCs (Abrahamsson et al., 2012) to allow direct assessment of possible developmental differences. We confirmed that 98% of the estimated diameters are larger than the imaging resolution (0.27 μm). We observed only a small developmental difference in the mean FWHM (0.41 vs. 0.47 μm, 13% reduction) using this approach. Because the dendritic filtering is similar for diameters ranging from 0.3 to 0.6 μm (Figure 4G and 4H, Abrahamsson et al. 2012), we concluded that developmental changes in dendritic diameter cannot account for for developmental differences in mEPSC time course.
We added the following text to the methods (line 777):
“The imaging resolution within the molecular layer was estimated from the width of intensity line profiles of SC axons. The FWHM was 0.30 +/- 0.01 μm (n = 57 measurements over 16 axons) and a mean of 0.27 +/- 0.01 μm (n = 16) when taking into account the thinnest section for each axon. Only 2% of all dendritic measurements are less than 270 nm, suggesting that the dendritic diameter estimation is hardly affected by the resolution of our microscope”
Regarding additional morphometrics:
- We added two panels (H and I) to Figure 6 showing the number of primary dendrites and branch points for immature and adult using the same estimation criteria as Myoga et al;,
- We have updated the Results section (line 389). “Thus, the larger number of puncta located further from the soma in adult SCs is not due to increased puncta density with distance, but a larger dendritic lengths (Figure 6E and 6F) and many more distal dendritic branches (Figure 6G, Sholl analysis) due to a larger number of branch points (Figure 6H), but not a larger number of primary dendrites (Figure 6I). The similarity between the shapes of synapse (Figure 6B) and dentric segment (Figure 6C) distributions was captured by a similarity in their skewness (0.38 vs. 0.32 for both distributions in immature and -0.10 and -0.08 for adult distributions). These data demonstrate that increased dendritic complexity during SC maturation is responsible for a prominent shift toward distal synapses in adult SCs.
As suggested by the reviewer, we estimated the dendritic width as a function branch order and observed a small reduction of dendritic segments as a function of distance from the soma that does not significantly alter the dendritic filtering (0.35 to 0.6 μm): there is a tendency to observe smaller diameter for more distal segments.
We also show the variability in dendritic diameter within single SCs and between different SCs, which can be very large. These results have been added to Figure 2B. See also point one below in response to “comment to authors.”
We will upload the two SC reconstructions to ModelDB.
- The Discussion should justify the assumption of AMPA-only synapses in the model (by citing available experimental data) as well as the limitations of this assumption in the case of different spatiotemporal patterns of parallel fiber activation.
NMDARs are extrasynaptic in immature and adult SCs. Therefore they do not contribute to postsynaptic strength in response to low-frequency synaptic activation. We therefore do not consider their contribution to synaptic integration in this study. Please see also out detailed response to reviewer’s point 4. We have updated the Results accordingly.
- What is the likely influence of gap junction coupling between SCs on the results presented here, and on synaptic integration in SCs more generally - and how does it change during development? This should also be discussed.
Please see a detailed response to Editor’s point 2. In brief, all recordings were performed without perturbing gap junction coupling between cells, which have been shown to affect axial resistance and membrane capacitance in other cell types (Szoboszlay et al., 2016). While our simulations do not explicitly include gap junctions, their effect on passive membrane properties is implicitly included because we matched the simulated membrane time constant to experimental values. Moreover, gap junctions are more prominent in cerebellar basket cells than SCs in both p18 to p21 animals (Rieubland 2015) and adult mice (Hoehne et al., 2020). Ultimately, the impact of gap junctions also depends on their distance from the activated synapses (Szoboszlay et al., 2016). Unfortunately, the distribution of gap junctions in SCs and their conductance is not known at this time. We, therefore, did not explicitly consider gap junction in this study.
Nevertheless, we have added a section in the Discussion (line 552):
“We cannot rule out that developmental changes in gap junction expression could contribute to the maturation of SC dendritic integration, since they are thought to contribute to the axial resistivity and capacitance of neurons (Szoboszlay et al., 2016). All the recordings were made with gap junctions intact, including for membrane time constant measurements. However, their expression in SCs is likely to be lower than their basket cell counterparts (Hoehne et al., 2020; Rieubland et al., 2014).”
- All experiments and all simulations in the manuscript were done in voltage clamp (the Methods section should give further details, including the series resistance). What is the significance of the key results of the manuscript on synapse distribution and branching pattern of postsynaptic dendrites in immature and adult SCs for the typical mode of synaptic integration in vivo, i.e. in current clamp? What is their significance for neuronal output, considering that SCs are spontaneously active?
It should be noted that not all simulations were done in voltage-clamp, see figure 8.
Nevertheless, we have given additional details about the following experimental and simulation parameters:
Description of the whole-cell voltage-clamp procedure.
Series resistance values of experiments and used for simulations.
Initial simulations with the idealized SC model were performed with a Rs of 20 MOhm. In the reconstructed model Rs was set at 16 mOhm to match more precisely the experimental values obtained for the mEPSC experiments. We verified that there were no statistical difference in Rs between Immature and adult recordings.
Reviewer #3 (Public Review):
- Although the authors were thorough in their efforts to find the mechanism underlying the differences in the young and adult SC synaptic event time course, the authors should consider the possibility of inherently different glutamate receptors, either by alterations in the subunit composition or by an additional modulatory subunit. The literature actually suggests that this might be the case, as several publications described altered AMPA receptor properties (not just density) during development in stellate cells (Bureau, Mulle 2004; Sun, Liu 2007; Liu, Cull-Candy 2002). The authors need to address these possibilities, as modulatory subunits are known to alter receptor kinetics and conductance as well.
Properties of synaptic AMPAR in SCs are known to change during development and in an activity-dependent manner. EPSCs in immature SC have been shown to be mediated by calcium permeable AMPARs, predominantly containing GluR3 subunits that are associated with TARP γ2 and γ7 (Soto et al. 2007; Bats et al., 2012). During development GluR2 subunits are inserted to the synaptic AMPAR in an activity-dependent manner (Liu et al, 2000), affecting the receptors’ calcium permeability (Liu et al., 2002). However, those developmental changes do not appear to affect EPSC kinetics (Liu et al., 2002) and have very little impact on AMPAR conductance (Soto et al., 2007). When we compare qEPSC kinetics for somatic synapses between immature and adult SC, we did not observe changes in EPSC decay. In the light of this observation and also consistent with the studies cited above, we concluded that differences in AMPAR composition could not contribute to kinetics differences observed in the developmental changes in mEPSC properties.
We have modified the manuscript to make this point clearer (see section starting line 332) :
“This reduction in synaptic conductance could be due to a reduction in the number of synaptic AMPARs activated and/or a developmental change in AMPAR subunits. SC synaptic AMPARs are composed of GluA2 and GluA3 subunits associated with TARP γ2 and γ7 (Bats et al., 2012; Liu and Cull-Candy, 2000; Soto et al., 2007; Yamazaki et al., 2015). During development, GluR2 subunits are inserted to the synaptic AMPAR in an activity-dependent manner (Liu and Cull-Candy, 2002), affecting receptors calcium permeability (Liu and Cull-Candy, 2000). However, those developmental changes have little impact on AMPAR conductance (Soto et al., 2007), nor do they appear to affect EPSC kinetics (Liu and Cull-Candy, 2002); the latter is consistent with our findings. Therefore the developmental reduction in postsynaptic strength most likely results from fewer AMPARs activated by the release of glutamate from the fusion of a single vesicle. “
The authors correctly identify the relationship between local dendritic resistance and the reduction of driving force, but they assume the same relationship for young SCs as well in their model. This assumption is not supported by recordings, and as there are several publications about the disparity of input impedance for young versus adult cells (Schmidt-Hieber, Bischoffberger 2007).
The input resistance of the dendrite will indeed determine local depolarization and loss of driving force. However, its impact on dendritic integration depends on it precise value, and perhaps the reviewer thought we “assumed” that the input resistance to be the same between immature and adult SCs. This was not the case, and we have since clarified this in the manuscript. We performed three important measurements that support a loss of driving force in immature SCs (for reference, the input resistance for an infinite cable is described by the following equation (Rn= sqrt(RmRi/2)/(2pi*r^(3/2)), where r is the dendrite radius):
The input resistance is inversely proportional to the dendritic diameter, which we measured to be only slightly larger in immature SCs (0.47 versus 0.41 μm). This result is described in Figure 2.
We measured the membrane time constant, which provides an estimate of the total membrane conductance multiplied by the total capacitance. The values between the two ages were similar, suggesting a slightly larger membrane resistance to compensate the smaller total membrane capacitance of the immature SCs. This was explicitly accounted for when performing the simulations using reconstructed immature and adult SCs (Figure 2 and 7 and 8) by adjusting the specific membrane resistance until the simulated membrane time constant matched experimental values. These values were not clearly mentioned and are now included on line 233 in the Results and 704 in the Methods.
We directly examined paired-pulse facilitation of synapses onto immature SC dendrites versus that for somatic synapses. We previously showed in adult SCs that sublinear summation of synaptic responses, due to loss of synaptic current driving force (Tran- Van-Minh et al. 2016), manifests in decreased facilitation for dendritic synapses (Abrahamsson et al. 2012). Figure 8A shows that indeed dendritic facilitation was less than observed in the soma. We have now modified Figure 8 to include the results of the simulations showing that the biophysical model could reproduce this difference in shortterm plasticity (Figure 8B).
Together, we believe these measurements support the presence of similar sublinear summation mechanisms in immature SCs.
- The authors use extracellular stimulation of parallel fibers. The authors note that due to the orientation of the PF, and the slicing angle, they can restrict the spatial extent of the stimuli. However, this method does not guarantee that the stimulated fibers will all connect to the same dendritic branch. Whether two stimulated synapses connect to the same dendrite or not can heavily influence summation. This is especially a great concern for these cells as the Scholl analysis showed that young and adult SC cells have different amount of distal dendrites. Therefore, if the stimulated axons connect to several different neighboring dendrites instead of the one or two in case of young SC cells, then the model calculations and the conclusions about the summation rules may be erroneous.
We selected isolated dendrites and delivered voltage stimuli using small diameter glass electrodes (~ 1 μm) 10 - 15 V above threshold to stimulate single dendrites. This procedure excites GC axons in brain slices made from adult mice within less than 10 μm from the tip (Figure 2C, Tran-Van-Minh et al. 2016). It produces large dendritic depolarizations that are sufficient to decrease synaptic current driving force (Figure 1, Tran-Van-Minh et al. 2016). When we reproduced the conductance ratio using uncaging of single dendrites, we observed paired-pulse facilitation in the dendrites – suggesting that electrical stimulation activated synapses on common dendritic branches, or at least within close electrotonic distance to cause large dendritic depolarizations (Figure 7, Abrahamsson et al. 2012). Finally, we expect that the decreased branching in immature SCs further ensures that a majority of recorded synapses are contacting a common dendritic segment. We cannot rule out that occasionally some synaptic responses recorded at the soma are from synapses on different dendritic branches, but we do not see how this would alter our results and change our principal conclusions, particularly since this possible error only effects the interpretation of how many synapses are activated in paired-pulse experiments. The majority of the conclusions arise from the stimulation of single vesicle release events, and given the strikingly perpendicular orientation of GC axons, a 10 μm error in synapse location along a dendrite when we stimulated in the outthird would not alter our interpretations of the data.
-

Evaluation Summary:
Using a range of cutting-edge techniques, the authors of this manuscript explore the functional consequences of developmental changes in dendritic morphology and synapse distribution in a GABAergic interneuron. The experiments are carefully performed and represent a thorough investigation of the structural and functional changes in synaptic inputs and postsynaptic dendritic branching patterns, an important topic for both developmental neurobiologists and synaptic physiologists. The data support most conclusions, but alternative interpretations remain possible and should be further considered.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the …
Evaluation Summary:
Using a range of cutting-edge techniques, the authors of this manuscript explore the functional consequences of developmental changes in dendritic morphology and synapse distribution in a GABAergic interneuron. The experiments are carefully performed and represent a thorough investigation of the structural and functional changes in synaptic inputs and postsynaptic dendritic branching patterns, an important topic for both developmental neurobiologists and synaptic physiologists. The data support most conclusions, but alternative interpretations remain possible and should be further considered.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
Here the authors use a variety of sophisticated approaches to assess the contribution of synaptic parameters to dendritic integration across neuronal maturation. They provide high-quality data identifying cellular parameters that underlie differences in AMPAR-mediated synaptic currents measured between adolescent and adult cerebellar stellate cells, and conclude that differences are attributed to an increase in the complexity of the dendritic arbor. This conclusion relies primarily on the ability of a previously described model for adult stellate cells to recapitulate the age-dependent changes in EPSCs by a change in dendritic branching with no change in synapse density. These rigorous results have implications for understanding how changing structure during neuronal development affects integration of …
Reviewer #1 (Public Review):
Here the authors use a variety of sophisticated approaches to assess the contribution of synaptic parameters to dendritic integration across neuronal maturation. They provide high-quality data identifying cellular parameters that underlie differences in AMPAR-mediated synaptic currents measured between adolescent and adult cerebellar stellate cells, and conclude that differences are attributed to an increase in the complexity of the dendritic arbor. This conclusion relies primarily on the ability of a previously described model for adult stellate cells to recapitulate the age-dependent changes in EPSCs by a change in dendritic branching with no change in synapse density. These rigorous results have implications for understanding how changing structure during neuronal development affects integration of AMPR-mediated synaptic responses.
The data showing that younger SCs have smaller dendritic arbors but similar synapse density is well-documented and provides compelling evidence that these structural changes affect dendritic integration. But the main conclusion also relies on the assumption that the biophysical model built for adult SCs applies to adolescent SCs, and there are additional relevant variables related to synaptic function that have not been fully assessed. Thus, the main conclusions would be strengthened and broadened by additional experimental validation.
-

Reviewer #2 (Public Review):
This manuscript investigates the cellular mechanisms underlying the maturation of synaptic integration in molecular layer interneurons in the cerebellar cortex. The authors use an impressive combination of techniques to address this question: patch-clamp recordings, 2-photon and electron microscopy, and compartmental modelling. The study builds conceptually and technically on previous work by these authors (Abrahamsson et al. 2012) and extends the principles described in that paper to investigate how developmental changes in dendritic morphology, synapse distribution and strength combine to determine the impact of synaptic inputs at the soma.
1. Models are constructed to confirm the interpretation of experimental results, mostly repeating the simulations from Abrahamsson et al. (2012) using 3D reconstructed …
Reviewer #2 (Public Review):
This manuscript investigates the cellular mechanisms underlying the maturation of synaptic integration in molecular layer interneurons in the cerebellar cortex. The authors use an impressive combination of techniques to address this question: patch-clamp recordings, 2-photon and electron microscopy, and compartmental modelling. The study builds conceptually and technically on previous work by these authors (Abrahamsson et al. 2012) and extends the principles described in that paper to investigate how developmental changes in dendritic morphology, synapse distribution and strength combine to determine the impact of synaptic inputs at the soma.
1. Models are constructed to confirm the interpretation of experimental results, mostly repeating the simulations from Abrahamsson et al. (2012) using 3D reconstructed morphologies. The results are as expected from cable theory, given the (passive) model assumptions. While this confirmation is welcome and important, it is disappointing to see the opportunity missed to explore the implications of the experimental findings in greater detail. For instance, with the observed distributions of synapses, are there more segregated subunits available for computation in adult vs immature neurons? How do SCs respond at different developmental stages with in vivo-like patterns of input, rather than isolated activation of synapses? Answering these sorts of questions would provide quantitative support for the conclusion that computational properties evolve with development.
2. From a technical perspective, the modeling appears to be well-executed, though more methodological detail is required for it to be reproducible. The AMPA receptor model and reversal potential are unspecified, as is the procedure for fitting the kinetics to data. Were simulations performed at resting potential, and if yes, what was the value? How was the quality of the morphological reconstructions assessed? Accurate measurement of dendritic diameters is crucial to the simulations in this study, so providing additional morphometrics would be helpful for assessing the results. Will the models and morphologies be deposited in ModelDB or similar?
3. The Discussion should justify the assumption of AMPA-only synapses in the model (by citing available experimental data) as well as the limitations of this assumption in the case of different spatiotemporal patterns of parallel fiber activation.
4. What is the likely influence of gap junction coupling between SCs on the results presented here, and on synaptic integration in SCs more generally - and how does it change during development? This should also be discussed.
5. All experiments and all simulations in the manuscript were done in voltage clamp (the Methods section should give further details, including the series resistance). What is the significance of the key results of the manuscript on synapse distribution and branching pattern of postsynaptic dendrites in immature and adult SCs for the typical mode of synaptic integration in vivo, i.e. in current clamp? What is their significance for neuronal output, considering that SCs are spontaneously active?
-

Reviewer #3 (Public Review):
- Although the authors were thorough in their efforts to find the mechanism underlying the differences in the young and adult SC synaptic event time course, the authors should consider the possibility of inherently different glutamate receptors, either by alterations in the subunit composition or by an additional modulatory subunit. The literature actually suggests that this might be the case, as several publications described altered AMPA receptor properties (not just density) during development in stellate cells (Bureau, Mulle 2004; Sun, Liu 2007; Liu, Cull-Candy 2002). The authors need to address these possibilities, as modulatory subunits are known to alter receptor kinetics and conductance as well.
The authors correctly identify the relationship between local dendritic resistance and the reduction of …
Reviewer #3 (Public Review):
- Although the authors were thorough in their efforts to find the mechanism underlying the differences in the young and adult SC synaptic event time course, the authors should consider the possibility of inherently different glutamate receptors, either by alterations in the subunit composition or by an additional modulatory subunit. The literature actually suggests that this might be the case, as several publications described altered AMPA receptor properties (not just density) during development in stellate cells (Bureau, Mulle 2004; Sun, Liu 2007; Liu, Cull-Candy 2002). The authors need to address these possibilities, as modulatory subunits are known to alter receptor kinetics and conductance as well.
The authors correctly identify the relationship between local dendritic resistance and the reduction of driving force, but they assume the same relationship for young SCs as well in their model. This assumption is not supported by recordings, and as there are several publications about the disparity of input impedance for young versus adult cells (Schmidt-Hieber, Bischoffberger 2007).
- The authors use extracellular stimulation of parallel fibers. The authors note that due to the orientation of the PF, and the slicing angle, they can restrict the spatial extent of the stimuli. However, this method does not guarantee that the stimulated fibers will all connect to the same dendritic branch. Whether two stimulated synapses connect to the same dendrite or not can heavily influence summation. This is especially a great concern for these cells as the Scholl analysis showed that young and adult SC cells have different amount of distal dendrites. Therefore, if the stimulated axons connect to several different neighboring dendrites instead of the one or two in case of young SC cells, then the model calculations and the conclusions about the summation rules may be erroneous.
-