Metal microdrive and head cap system for silicon probe recovery in freely moving rodent
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The manuscript describes an improved methodology for performing electrophysiological experiments (involving recording of the activity of tens or hundreds of neurons in the brain simultaneously) in freely behaving mice and rats using silicon probes. By providing a versatile microdrive and head cap design for rodents, this paper may contribute to ease silicon probe chronic recording and recovery, thus reducing experimental costs and making the technique more accessible. The paper is expected to appeal to a broad range of systems neuroscientists who seek to understand how the brain commands movement and behavior.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
High-yield electrophysiological extracellular recording in freely moving rodents provides a unique window into the temporal dynamics of neural circuits. Recording from unrestrained animals is critical to investigate brain activity during natural behaviors. The use and implantation of high-channel-count silicon probes represent the largest cost and experimental complexity associated with such recordings making a recoverable and reusable system desirable. To address this, we have designed and tested a novel 3D printed head-gear system for freely moving mice and rats. The system consists of a recoverable microdrive printed in stainless steel and a plastic head cap system, allowing researchers to reuse the silicon probes with ease, decreasing the effective cost, and the experimental effort and complexity. The cap designs are modular and provide structural protection and electrical shielding to the implanted hardware and electronics. We provide detailed procedural instructions allowing researchers to adapt and flexibly modify the head-gear system.
Article activity feed
-

Author Response:
Reviewer #1:
In this ms, Voroslakos et al., describe a customizable and versatile microdrive and head cap system for silicon probe recordings in freely moving rodents (mice and rats). While there are similar designs elsewhere, the added value here is: a) a carefully designed solution to facilitate probe recovery, thus reducing experimental costs and favoring reproducibility; b) flexibility to accommodate several microdrives and additional instrumentation; c) open access design and documentation to favor customization and dissemination. Authors provide detailed description to faccilitate building the system.
Personally, I found this resource very useful to democratize multi-site recordings, not only for standard silicon probes, but also more novel integrated optoelectrodes and neuropixels. While there are other …
Author Response:
Reviewer #1:
In this ms, Voroslakos et al., describe a customizable and versatile microdrive and head cap system for silicon probe recordings in freely moving rodents (mice and rats). While there are similar designs elsewhere, the added value here is: a) a carefully designed solution to facilitate probe recovery, thus reducing experimental costs and favoring reproducibility; b) flexibility to accommodate several microdrives and additional instrumentation; c) open access design and documentation to favor customization and dissemination. Authors provide detailed description to faccilitate building the system.
Personally, I found this resource very useful to democratize multi-site recordings, not only for standard silicon probes, but also more novel integrated optoelectrodes and neuropixels. While there are other solutions, this design is quite simple and versatile. A potential caveat is whether it could be perceived as just an upgrade, given some similitudes with previous designs (e.g. Chung et al., Sci Rep 2017 doi: 10.1038/s41598-017-03340-5) and concepts (Headley et al., JNP doi: 10.1152/jn.00955.2014). However, the system presented in this paper provides added value and knowledge-based solutions to make silicon probe recordings more accessible.
We thank the reviewer for carefully reading our manuscript and providing useful and constructive comments.
Reviewer #2:
This manuscript provides an updated guide on the procedures for performing chronic recordings with silicon probes in mice and rats in the lab of the senior author, who is one of the leaders in the use of this experimental method. The new set of procedures relies on metal and plastic 3D printed parts, and represents a major improvement over the older methodology (i.e. Vandecasteele et al. 2012).
The manuscript is clearly written and the technical instructions (in the Methods section) seem rather detailed. The main concerns I had are as follows.
We thank the reviewer for carefully reading our manuscript and providing useful and constructive comments.
- The present design is an improvement over Chung et al. (the most similar previously published explantable microdrive design, as far as I am aware) in terms of the footprint and travel distance. However, a main disadvantage of the system in its present form is that (apparently) it does not support Neuropixels probes. While such probes might not be suitable for some uses (e.g. to record from large populations in dorsal hippocampus), Neuropixels probes are of considerable interest to many labs.
Our microdrive and head cap system can also support Neuropixels probes. Since our initial submission, we have implanted a Neuropixels probe in the intermediate hippocampus of a rat using our recoverable, plastic microdrive. At the end of the experiment, the Neuropixels probe was successfully recovered, cleaned, and implanted again in a new rat. In addition, we designed a new arm for our metal microdrive which can support Neuropixels probes (Figure 2) and implanted another rat (Figure 3 and 4). We have also created a video showing how to attach Neuropixels probe to a metal microdrive (Suppl. Video 3).
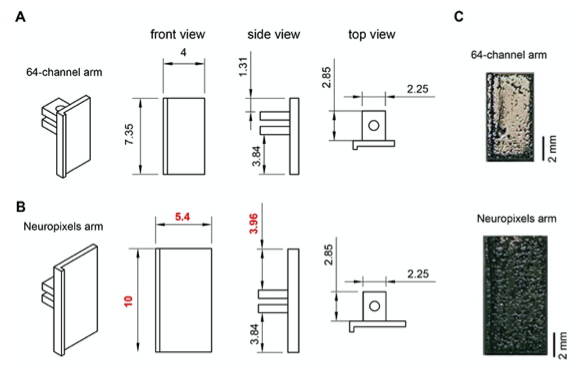
Figure 2. Metal microdrive adapter for Neuropixels probe. A Arm design for 64-channel silicon probes. 45o, front, side and top views are shown (from left to right). All dimensions are in mm. B Changing the overall length (from 7.35 mm to 10 mm) and width (from 4 mm to 5.4 mm) of the 64-channel arm makes our metal microdrive compatible with Neuropixels probe. Note, that only three dimensions of the 64-channel arm were modified (red numbers). 45-degree, front, side and top views are shown (from left to right). All dimensions are in mm. C Photograph of the different arm designs of the metal, recoverable microdrive (top shows an arm designed for a 64-ch silicon probe, bottom shows an arm designed for Neuropixels probe).
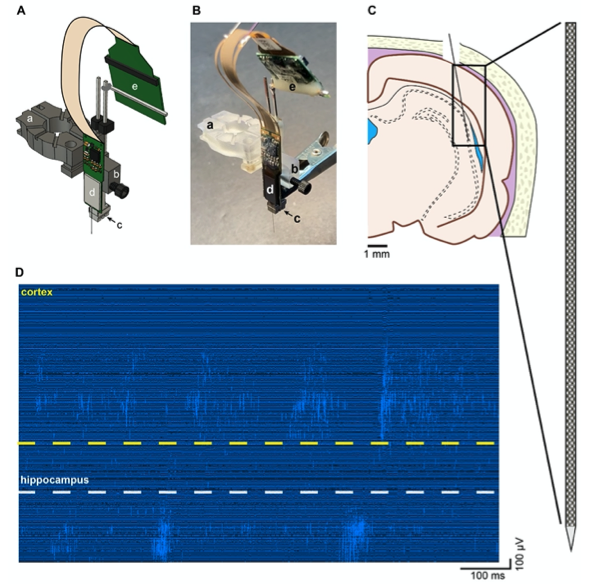
Figure 3. Recording of unit firing with Neuropixels probe attached to a metal microdrive in freely moving rat. A Metal microdrive for Neuropixels probe (a – stereotax attachment, b – drive holder, c – metal microdrive, d – Neuropixels probe and e – Neuropixels headstage). B Photo of Neuropixels probe attached to a metal microdrive (a-e same as in A). C Location of probe implantation (Bregma - 4.8 mm, mediolateral + 4.6 mm, 11-degree angle). D High pass filtered traces (1s) from a freely moving rat implanted with Neuropixels probe. Note the single unit activity in the cellular layer of cortex (top) and hippocampus (bottom).
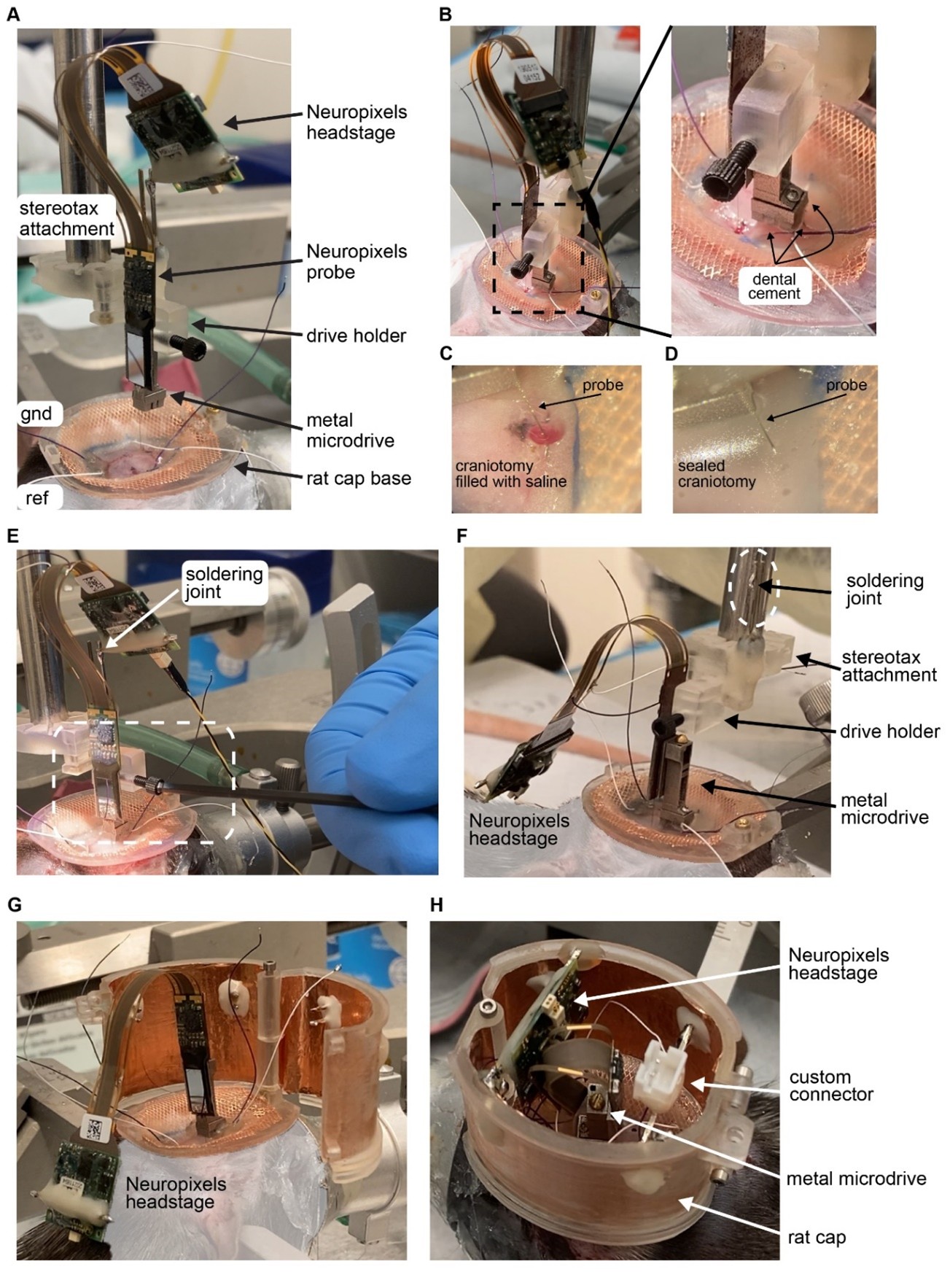
Figure 4. Implantation of Neuropixels probe in a rat using metal microdrive and rat cap system. A The base of the rat cap is attached to the skull. Reference (ref) and ground (gnd) screws are placed over the cerebellum. Neuropixels probe is mounted on a metal microdrive. The microdrive is held by the drive holder and attached to a stereotax arm using the stereotax attachment. For more details, see video: Neuropixels_attachment.mp4. B Once the probe is inserted to its final depth (left), the base of the microdrive is cemented to the skull (zoomed in photograph on the right). C The surface of the brain is kept wet using saline during probe insertion and during cementing the base of the microdrive. D After the base is cemented, the craniotomy is sealed with bone wax. E Releasing the drive from the drive holder. Once it is released the stereotax arm is moved upwards. F Neuropixels headstage is removed from the male header of the stereotax attachment (soldering joint) and placed on the animals back. G The walls of the cap system are attached to the base. Ground and reference wires are soldered to the probe (not shown). H The male header of the headstage is secured to the walls. The headstage and its cable are oriented to allow easy access to the screw head of the microdrive. Note, that there is enough room for custom connectors inside the rat cap.
- The total weight of the mouse implant seems quite high (together with the headstage, I estimate it is >= 4gr). Could the authors provide the exact value, and describe whether this has any impact on the way the animal moves? Also, the authors should describe how the animals are housed (e.g. do they carry the headstage even when not being recorded). The authors say that a mouse can be implanted with more than one microdrive. The authors should clarify whether they actually have an experience with such implants, or is this just a suggestion based on their educated estimate?
The total weight of the metal microdrive, including the base, body and arm is 0.87 gram. Additional weight is the metabond and dental acrylic cement. The amount of cement that is used during surgery can vary between researchers and the type of surgery. The overall weight of the assembly also depends on the silicon probe with Omnetics connector(s) that is used for the surgery, e.g.: 32-channel micro-LED probe is 1.11g (NeuroLight Technologies LTD.), 64-channel 4-shank probe is 0.96g (ASSY E-1, Cambridge NeuroTech), 64-channel 5-shank probe is 1.05g (A5x12- 16-Buz-Lin-5mm, NeuroNexus Ltd.) and a 128-channel 4-shank probe with integrated Intan chips is 0.94g (P128-5, Diagnostic Biochips). In addition, the overall weight of the entire assembly can change if optic fibers are used in optogenetic studies or if any custom connectors are implanted (e.g., connector and wires for brain stimulation). That is the reason why we reported the overall weight of each system (metal microdrive, mouse cap and rat cap) individually.
The implanted mice are single housed, and they do not carry the headstage while in the vivarium. During recordings, the headstage is attached and a counterbalanced pulley system ensures that the animal is not carrying the extra weight of the headstage. We have quantitatively compared running speed with traditional and the new head caps in both rats and mice (Fig. 6).
The small footprint of the metal microdrive enables researchers to perform more than one silicon probe implantation in freely moving mice. For this purpurse, larger mice (>35 g) are selected (Figure 5).
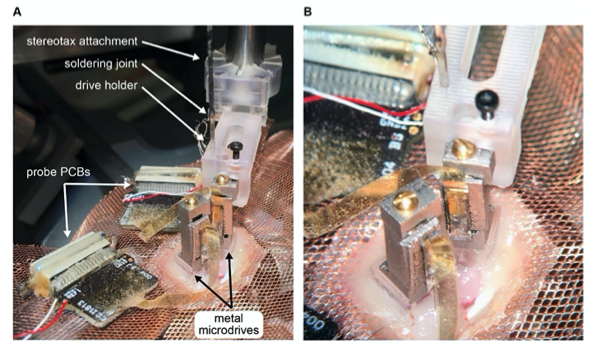
Figure 5. Metal microdrive enables double silicon probe recordings in freely moving mice. A Intraoperative photograph of double silicon probe implantation. Note that the metal microdrive on the left had been secured to the skull and the second drive is being implanted using the stereotaxic attachment and drive holder. The probe PCBs are placed on the copper mesh. B Photograph focused on the metal microdrives.
- There is no information in the results section on the number of implants performed, the duration the animals were implanted, the quality of the recordings obtained, number of successes or failures failures. The figures merely provide examples of one successful recording in a mouse and in a rat. All these details should be provided, along with details of how many probes were reused and how many times (a brief mention of one case, lines 252-253 and 359-360, is not sufficient).
We have added a Supplementary Table explaining all the details of our implants. We would like to refer the Reviewer to response #1 to Reviewer 1.
Adapting new technology is challenging. To date, we have extensive experience with the rat cap system only (n=3 users in the lab, n = 25 rats implanted). Two lab members have started to adapt our mouse cap and implanted 3 mice since our submission. We included their maze running behavioral data for comparison between the copper mesh and cap system.
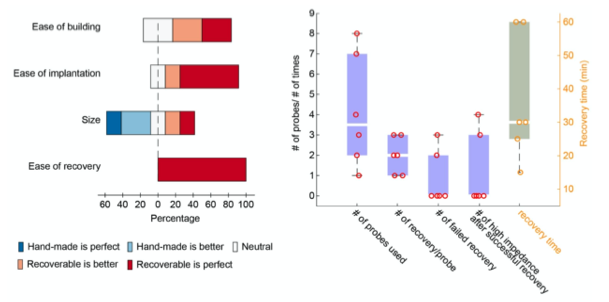
Prior to the development of the metal microdrive, we have conducted an internal lab survey comparing the hand-made microdrive (Vandecasteele et al., 2012) and our recoverable, plastic microdrive. Six lab members who had extensive experience with both types participated (Figure 6). Our questions were:
- On a scale 1-10, how would you compare the plastic, recoverable drive to the Vandecasteele et. al. 2012 one in terms of: a) ease of building a drive, b) size and c) ease of recovery.
Figure 6. Internal lab survey using recoverable, plastic microdrives. A User feedback based on four criteria: ease of building, ease of implantation, size, ease of recovery. The 3D printed microdrive surpasses the manually built drive (Vandecasteele et. al., 2012) on every parameter except the size. B 24 silicon probes were used with the recoverable plastic microdrive. On average each probe was recovered two times. Out of these 48 recovery attempts 5 failed only. There were 2 total losses during recovery and in three cases different number of shanks broke during the recovery process making the recovery partially successful. One major limitation of reusability is the sudden increase in impedance over time (we have to discard 30% of the successfully recovered probes due this reason). Researchers in our lab spend on average 30 minutes to recover a silicon probe.
Overall, the success rate of recovery is much higher using a recoverable microdrive system, but the size of the plastic, recoverable microdrive is limits certain experiments. This was one of the main motivations to develop the metal, recoverable microdrive.
- In fig. 2, spike waveforms are classified as pyramidal, wide or narrow interneurons. I did not find any description of how this classification was performed.
We have removed the single cell putative cell types from the manuscript as this issue is not relevant to the current manuscript. Figure 2 has been simplified and a new figure 5 is dedicated to the single cell quantification.
- Also in fig. 2, refractory period violations are reported in percent (permille in fact). First, it is not clear how refractory period was defined. Second, such quantification is incorrect in principle: we use refractory period violations to infer the rate of false positives. Yet the relationship between fraction of ISI violations and false positive rate depends on the firing rate of the neuron. For example, 0.1% of ISI violations is quite good for a unit spiking at 10 spikes/s, is so so for a unit spiking at 1 spike/s, and is very bad if the firing rate is 0.1 spike/s (see Hill et al. JNeurosci. 2011 for derivation). Alternatively, the authors can follow an approach described in an old paper by the same lab (Harris et al., JNeuropsysiol. 2000), quantifying the violations in spike autocorrelogram relative to its asymptotic height.
We have removed this panel from Fig. 2 and dedicated a new figure (Fig. 5) to the single cell quantification. Refractory violations can be used as an alarm for poor cluster quality. Absence of refractory violations alone does not guarantee good separation for the reasons the Reviewer mentioned.
- Line 477: the authors write that the probes were mounted on a plastic microdrive. This seems to contradict the key claim of the manuscript (namely that the microdrives were from stainless steel).
We apologize if this description was not clear in the original manuscript. In the revised version, we have added a table (Suppl. Table 1) explaining all details of each animal subject (species, strain, weight, cap type), type of silicon probe and microdrive used. As we explained in Response 3, our main goal was to test each system individually and once all components have been verified, we combined everything into one surgery.
The plastic and metal microdrives are based on the same principles. The implantation/recovery tools are also identical in design concepts. Based on our own experience, users dol not recognize any changes in terms of ease of use, ease of implantation and ease of recovery when changing from plastic recoverable microdrives to metal ones. The advantage of metal drives is size reduction, their multiple reusability and stability.
- I believe that the work of Luo & Bondy et al. (eLife 2020) and should be references and compared to.
We reference Luo et. al. (2020) in our revised manuscript. One of the main advantages of using a microdrive system is the ability to move the recording probe inside the brain tissue and sample new sets of neurons. This is not the case in Luo & Bondy et al. (eLife 2020).
-

Reviewer #3 (Public Review):
In this paper, the authors develop a method and device to use electrophysiology recording arrays in freely moving rodents. Such methods will empower more researchers to use these recording devices in their labs and ultimately save significant time and resources in developing such methods on their own.
The strengths of this paper are its very clear explanation of the protocol (in both writing, images, and a video) and the device that they developed. With the detailed instructions provided here, other researchers will readily be able to adapt this protocol in their own labs. The device is reproducible using common 3D printing services and can be easily modified thanks to its CAD format. There are a few areas in which the written protocol could be improved, but these will be easy to address. Overall, the way …
Reviewer #3 (Public Review):
In this paper, the authors develop a method and device to use electrophysiology recording arrays in freely moving rodents. Such methods will empower more researchers to use these recording devices in their labs and ultimately save significant time and resources in developing such methods on their own.
The strengths of this paper are its very clear explanation of the protocol (in both writing, images, and a video) and the device that they developed. With the detailed instructions provided here, other researchers will readily be able to adapt this protocol in their own labs. The device is reproducible using common 3D printing services and can be easily modified thanks to its CAD format. There are a few areas in which the written protocol could be improved, but these will be easy to address. Overall, the way that the authors have presented their protocols with such clarity and detail should serve as a precedent for future papers like this.
However, this paper is lacking transparency around several aspects: how often the use of this device is successful, with what kinds of recording devices it can be used, and how animals behave with the device implanted. The manuscript would be significantly strengthened by a clear explanation of how many neurons can be recorded with which probes, and the stability of these recordings over time. It would be beneficial for experimenters planning on using these devices to know which recording probes can be used as well as the chances of successfully recovering the probe. Lastly, there is limited description of how animals manage the weight of the entire device after implantation. Describing this with more detail would be very useful to researchers who wish to use these devices for freely moving behaviors that require quick, unimpeded movement.
Ultimately, this paper will provide an easily adaptable method for other researchers to use in their labs. This will save other researchers significant time and will enable more efficient and reproducible freely moving electrophysiology experiments.
-

Reviewer #2 (Public Review):
This manuscript provides an updated guide on the procedures for performing chronic recordings with silicon probes in mice and rats in the lab of the senior author, who is one of the leaders in the use of this experimental method. The new set of procedures relies on metal and plastic 3D printed parts, and represents a major improvement over the older methodology (i.e. Vandecasteele et al. 2012).
The manuscript is clearly written and the technical instructions (in the Methods section) seem rather detailed. The main concerns I had are as follows.
The present design is an improvement over Chung et al. (the most similar previously published explantable microdrive design, as far as I am aware) in terms of the footprint and travel distance. However, a main disadvantage of the system in its present form is that …
Reviewer #2 (Public Review):
This manuscript provides an updated guide on the procedures for performing chronic recordings with silicon probes in mice and rats in the lab of the senior author, who is one of the leaders in the use of this experimental method. The new set of procedures relies on metal and plastic 3D printed parts, and represents a major improvement over the older methodology (i.e. Vandecasteele et al. 2012).
The manuscript is clearly written and the technical instructions (in the Methods section) seem rather detailed. The main concerns I had are as follows.
The present design is an improvement over Chung et al. (the most similar previously published explantable microdrive design, as far as I am aware) in terms of the footprint and travel distance. However, a main disadvantage of the system in its present form is that (apparently) it does not support Neuropixels probes. While such probes might not be suitable for some uses (e.g. to record from large populations in dorsal hippocampus), Neuropixels probes are of considerable interest to many labs.
The total weight of the mouse implant seems quite high (together with the headstage, I estimate it is >= 4gr). Could the authors provide the exact value, and describe whether this has any impact on the way the animal moves? Also, the authors should describe how the animals are housed (e.g. do they carry the headstage even when not being recorded). The authors say that a mouse can be implanted with more than one microdrive. The authors should clarify whether they actually have an experience with such implants, or is this just a suggestion based on their educated estimate?
There is no information in the results section on the number of implants performed, the duration the animals were implanted, the quality of the recordings obtained, number of successes or failures failures. The figures merely provide examples of one successful recording in a mouse and in a rat. All these details should be provided, along with details of how many probes were reused and how many times (a brief mention of one case, lines 252-253 and 359-360, is not sufficient).
In fig. 2, spike waveforms are classified as pyramidal, wide or narrow interneurons. I did not find any description of how this classification was performed.
Also in fig. 2, refractory period violations are reported in percent (permille in fact). First, it is not clear how refractory period was defined. Second, such quantification is incorrect in principle: we use refractory period violations to infer the rate of false positives. Yet the relationship between fraction of ISI violations and false positive rate depends on the firing rate of the neuron. For example, 0.1% of ISI violations is quite good for a unit spiking at 10 spikes/s, is so so for a unit spiking at 1 spike/s, and is very bad if the firing rate is 0.1 spike/s (see Hill et al. JNeurosci. 2011 for derivation). Alternatively, the authors can follow an approach described in an old paper by the same lab (Harris et al., JNeuropsysiol. 2000), quantifying the violations in spike autocorrelogram relative to its asymptotic height.
Line 477: the authors write that the probes were mounted on a plastic microdrive. This seems to contradict the key claim of the manuscript (namely that the microdrives were from stainless steel).
I believe that the work of Luo & Bondy et al. (eLife 2020) and should be references and compared to.
-

Reviewer #1 (Public Review):
In this ms, Voroslakos et al., describe a customizable and versatile microdrive and head cap system for silicon probe recordings in freely moving rodents (mice and rats). While there are similar designs elsewhere, the added value here is: a) a carefully designed solution to facilitate probe recovery, thus reducing experimental costs and favoring reproducibility; b) flexibility to accommodate several microdrives and additional instrumentation; c) open access design and documentation to favor customization and dissemination. Authors provide detailed description to faccilitate building the system.
Personally, I found this resource very useful to democratize multi-site recordings, not only for standard silicon probes, but also more novel integrated optoelectrodes and neuropixels. While there are other solutions, …
Reviewer #1 (Public Review):
In this ms, Voroslakos et al., describe a customizable and versatile microdrive and head cap system for silicon probe recordings in freely moving rodents (mice and rats). While there are similar designs elsewhere, the added value here is: a) a carefully designed solution to facilitate probe recovery, thus reducing experimental costs and favoring reproducibility; b) flexibility to accommodate several microdrives and additional instrumentation; c) open access design and documentation to favor customization and dissemination. Authors provide detailed description to faccilitate building the system.
Personally, I found this resource very useful to democratize multi-site recordings, not only for standard silicon probes, but also more novel integrated optoelectrodes and neuropixels. While there are other solutions, this design is quite simple and versatile. A potential caveat is whether it could be perceived as just an upgrade, given some similitudes with previous designs (e.g. Chung et al., Sci Rep 2017 doi: 10.1038/s41598-017-03340-5) and concepts (Headley et al., JNP doi: 10.1152/jn.00955.2014). However, the system presented in this paper provides added value and knowledge-based solutions to make silicon probe recordings more accessible.
-

Evaluation Summary:
The manuscript describes an improved methodology for performing electrophysiological experiments (involving recording of the activity of tens or hundreds of neurons in the brain simultaneously) in freely behaving mice and rats using silicon probes. By providing a versatile microdrive and head cap design for rodents, this paper may contribute to ease silicon probe chronic recording and recovery, thus reducing experimental costs and making the technique more accessible. The paper is expected to appeal to a broad range of systems neuroscientists who seek to understand how the brain commands movement and behavior.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 …
Evaluation Summary:
The manuscript describes an improved methodology for performing electrophysiological experiments (involving recording of the activity of tens or hundreds of neurons in the brain simultaneously) in freely behaving mice and rats using silicon probes. By providing a versatile microdrive and head cap design for rodents, this paper may contribute to ease silicon probe chronic recording and recovery, thus reducing experimental costs and making the technique more accessible. The paper is expected to appeal to a broad range of systems neuroscientists who seek to understand how the brain commands movement and behavior.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their names with the authors.)
-