Motor planning brings human primary somatosensory cortex into action-specific preparatory states
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The authors studied the neural correlates of planning and execution of single finger presses in a 7T fMRI study focusing on primary somatosensory (S1) and motor (M1) cortices. BOLD patterns of activation/deactivation and finger-specific pattern discriminability indicate that M1 and S1 are involved not only during execution, but also during planning of single finger presses. These results contribute to a developing story that the role of primary somatosensory cortex goes beyond pure processing of tactile information and will be of interest for researchers in the field of motor control and of systems neuroscience.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
- @MariusPeelen's saved articles (MariusPeelen)
Abstract
Motor planning plays a critical role in producing fast and accurate movement. Yet, the neural processes that occur in human primary motor and somatosensory cortex during planning, and how they relate to those during movement execution, remain poorly understood. Here, we used 7T functional magnetic resonance imaging and a delayed movement paradigm to study single finger movement planning and execution. The inclusion of no-go trials and variable delays allowed us to separate what are typically overlapping planning and execution brain responses. Although our univariate results show widespread deactivation during finger planning, multivariate pattern analysis revealed finger-specific activity patterns in contralateral primary somatosensory cortex (S1), which predicted the planned finger action. Surprisingly, these activity patterns were as informative as those found in contralateral primary motor cortex (M1). Control analyses ruled out the possibility that the detected information was an artifact of subthreshold movements during the preparatory delay. Furthermore, we observed that finger-specific activity patterns during planning were highly correlated to those during execution. These findings reveal that motor planning activates the specific S1 and M1 circuits that are engaged during the execution of a finger press, while activity in both regions is overall suppressed. We propose that preparatory states in S1 may improve movement control through changes in sensory processing or via direct influence of spinal motor neurons.
Article activity feed
-

-

Author Response:
Evaluation Summary:
The authors studied the neural correlates of planning and execution of single finger presses in a 7T fMRI study focusing on primary somatosensory (S1) and motor (M1) cortices. BOLD patterns of activation/deactivation and finger-specific pattern discriminability indicate that M1 and S1 are involved not only during execution, but also during planning of single finger presses. These results contribute to a developing story that the role of primary somatosensory cortex goes beyond pure processing of tactile information and will be of interest for researchers in the field of motor control and of systems neuroscience.
We thank all reviewers and the editor for their assessment of our paper. We acknowledge that our description of the methods and some interpretation of the results can be clarified and …
Author Response:
Evaluation Summary:
The authors studied the neural correlates of planning and execution of single finger presses in a 7T fMRI study focusing on primary somatosensory (S1) and motor (M1) cortices. BOLD patterns of activation/deactivation and finger-specific pattern discriminability indicate that M1 and S1 are involved not only during execution, but also during planning of single finger presses. These results contribute to a developing story that the role of primary somatosensory cortex goes beyond pure processing of tactile information and will be of interest for researchers in the field of motor control and of systems neuroscience.
We thank all reviewers and the editor for their assessment of our paper. We acknowledge that our description of the methods and some interpretation of the results can be clarified and expanded. We address every comment and proposed suggestion in the following below.
Reviewer #1 (Public Review):
This is a very important study for the field, as the involvement of S1 in motor planning has never been described. The paradigm is very elegant, the methods are rigorous and the manuscript is clearly written. However, there are some concerns about the interpretation of the data that could be addressed.
We thank Reviewer #1 for the positive evaluation of our study. We clarify our methodological choices and interpretation of the data in the following response.
• The authors claim that planning and execution patterns are scaled version of each other, and that overt movement during planning is prevented by global deactivation. This is an interesting perspective, however the presented data are not fully convincing to support this claim:
(1) the PCM analysis shows that correlation models ranging from 0.4 to 1 perform similarly to the best correlation model. This correlation range is wide and suggests that the correspondence between execution/planning patterns is only partial.
The reviewer is correct that the current data leaves us with a specific amount of uncertainty. However, it should be noted that the maximum-likelihood estimates of correlations between noisy patterns are biased, as they are constrained to be smaller or equal to 1. Thus, we cannot test the hypothesis that the correlation is 1 by just comparing correlation estimates to 1 (for details on this, see our recent blog on this topic: http://www.diedrichsenlab.org/BrainDataScience/noisy_correlation/). To test this idea, we therefore use a generative approach (the PCM analysis). We find that no correlation model has a higher log-likelihood than the 1-correlation model, therefore we cannot rule out that the underlying true correlation is actually 1. In other words, we have as much evidence that the correspondence is only partial as we do that the correspondence is perfect. The ambiguity given by the wide correlation range is due to the role of measurement noise in the data and should not be interpreted as if the true correlation was lower than 1. What we can confidently conclude is that activity patterns have a substantial positive correlation between planning and execution. We take this opportunity to clarify this point in the results section.
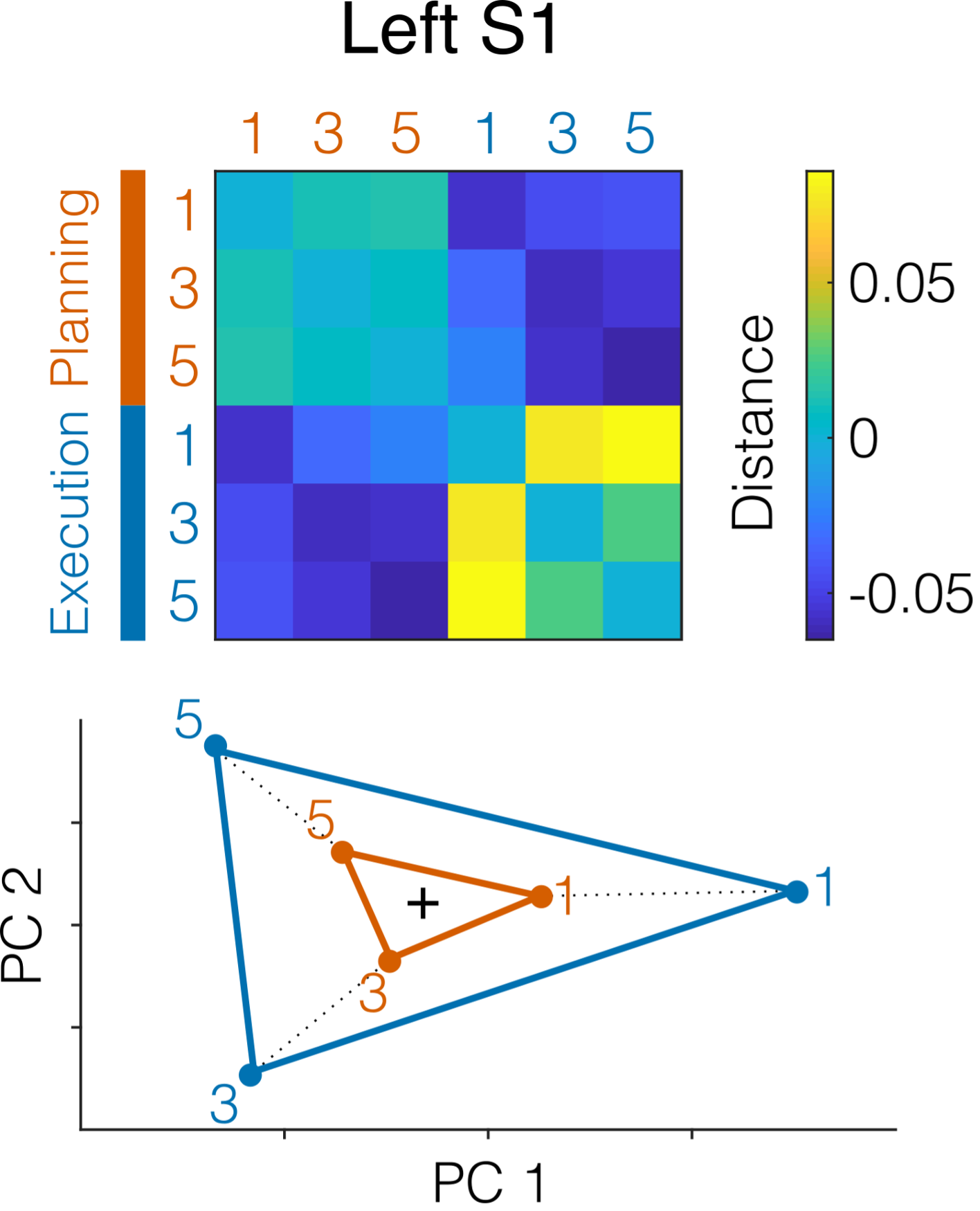
(2) in Fig.4 A-B, the distance between execution/planning patterns is much larger than the distance between fingers. How can such a big difference be explained if planning/execution correspond to scaled versions of the same finger-specific patterns? If the scaling is causing this difference, then different normalization steps of the patterns should have very specific effects on the observed results: 1) removing the mean value for each voxel (separately for execution and planning conditions) should nullify the scaling and the planning/execution patterns should perfectly align in a finger-specific way; 2) removing the mean pattern (separately for each finger conditions) should effectively disturb the finger-specific alignment shown in Fig.4C. These analyses would corroborate the authors' conclusion.
The large distance between planning and execution patterns (compared to the distance between fingers) is caused by the fact that the average activity pattern associated with planning differs substantially from the average activity pattern during execution. Such a large difference is of course expected, given the substantially higher activity during execution. However, here we are testing the hypothesis that the pattern vectors that are related to a specific finger within either planning or execution are scaled version of each other. Visually, this can be seen in Figure 4B (bottom), where the MDS plot is rotated, such the line of sight is in the direction of the mean pattern difference between planning and execution—such that it disappears in the projection. Relative to the baseline mean of the data (cross), you can see that arrangement of the fingers in planning (orange) is a scaled version of the arrangement during execution (blue). The PCM model provides a likelihood-based test for this idea. The model accounts for the overall difference between planning and execution by including (and estimating) model terms related to the mean pattern of planning and execution, respectively, therefore effectively removing the mean activation of planning and execution. We have now explained this better in the results and methods sections, also referring to a Jupyter notebook example of the correlation model used (https://pcm-toolbox-python.readthedocs.io/en/latest/demos/demo_correlation.html).
Regarding your analysis suggestions, removing the mean pattern for planning and execution across fingers as a fixed effect (suggestion 1) leads to the distance structure shown in Fig 4B (bottom)—showing that the finger-specific patterns during planning are scaled versions of those during execution (also see Fig. R1 below). On the other hand, subtracting the mean finger pattern across planning and execution (suggestion 2) will not fully remove the finger specific activation as the finger-specific patterns are differently scaled in planning and execution. Furthermore, neither of these subtraction analyses allows for a formal test of the hypotheses that the data can be explained by a pure scaling of the finger-specific patterns.
Figure R1. RDM of left S1 activity patterns evoked by the three fingers (1, 3, 5) during no-go planning (orange) and execution (blue) after removing the mean pattern across fingers (separately for planning and execution). The bottom shows the corresponding multidimensional scaling (MDS) projection of the first two principal components. Black cross denotes mean pattern across conditions.
• A conceptual concern is related to the task used by the authors. During the planning phase, as a baseline task, participants are asked to maintain a low and constant force for all the fingers. This condition is not trivial and can even be considered a motor task itself. Therefore, the planning/execution of the baseline task might interfere with the planning/execution of the finger press task. Even more controversial, the design of the motor task might be capturing transitions between different motor tasks (force on all finger towards single-finger press) rather than pure planning/execution of a single task. The authors claim that the baseline task was used to control for involuntary movements, however, EMG recordings could have similarly controlled for this aspect, without any confounds.
Participants received training the day before scanning, which made the “additional” motor task very easy, almost trivial. In fact, the system was calibrated so that the natural weight of the hand on the keys was enough to bring the finger forces within the correct range to be maintained. Thus, very little planning/online control was required by the participants before pressing the keys. As for the concern of capturing transitions between different motor tasks, that it is indeed always the case in natural behavior. Arguably there is no such thing as “pure rest” in the motor system, active effort has to be made even to maintain posture. Furthermore, if the motor system considers the hold phase as a simultaneous movement phase, it should have prevented M1 and S1 to participate in the planning of upcoming movements, as it would be busy with maintaining and controlling the pre-activation. Having found clear planning related signals in M1 and S1 in this situation makes our argument, if anything, stronger.
Finally, we specifically chose not to do EMG recordings because finger forces are a more sensitive measure of micro movements than EMG. Extensive pilot experiments for our papers studying ipsilateral representations and mirroring (e.g., Diedrichsen et al., 2012; Ejaz et al., 2018) have shown that we can pick up very subtle activations of hand muscles by measuring forces of a pre-activated hand, signals that clearly escape detection when recording EMG in the relaxed state. Based on these results, we actually consider the recording of EMG during the relaxed state as an insufficient control for the absence of cortical-spinal drive onto hand muscles. This is especially a concern when recording EMG during scanning, due to the decreased signal-to-noise ratio.
• In Fig.2F, the authors show no-planning related information in high-order areas (PMd, aSPL), while such information is found in M1 and S1. This null result from premotor and parietal areas is rather surprising, considering current literature, largely cited by the authors, pointing to high-order motor or parietal areas involved in action planning.
We agree with the reviewer that, to some extent, the lack of involvement of high-order areas in planning is surprising. However, we believe that task difficulty (i.e., planning demands) plays a role in the amount of observed planning activation. In other words, because participants were only asked to plan repeated movements of one finger, there was little to plan. The fact that this may have contributed to the null result in premotor and parietal areas was further confirmed by the second half of the dataset, which is not reported in the current paper. Here, we investigated the planning of multi-finger sequences, where planning demands are certainly higher. We found that high-order areas such as PMd and SPL were indeed active and involved in the planning of those, as expected. We decided to split the dataset across two publications as the multi-finger sequences have their own complexities, which would have distracted from the main finding of planning related activity in M1 and S1.
Reviewer #3 (Public Review):
I found the manuscript to be well written and the study very interesting. There are, however, some analytical concerns that in part arise because of a lack of clarity in describing the analyses.
- Some details regarding the methods used and results in the figures were missing or difficult to understand based on the brief description in the Methods section or figure legend.
We thank Reviewer #3 for pointing out some lack of clarity in our description of the methods. We now expanded both the methods section and the figure captions (Fig. 2-3-4).
- I think the manuscript would benefit from a more balanced description on the role of S1. As the authors state, S1 is traditionally thought to process afferent tactile and proprioceptive input. However, in the past years, S1 has been shown to be somatopically activated during touch observation, attempted movements in the absence of afferent tactile inputs, and through attentional shifts (Kikkert et al., 2021; Kuehn et al., 2014; Puckett et al., 2017; Wesselink et al., 2019). Furthermore, S1 is heavily interconnected with M1, so perhaps if such activity patterns are present in M1, they could also be expected in S1?
To better characterize the role of S1 during movement planning, we now include recent research showing that S1 can be somatotopically recruited even in the absence of tactile inputs.
- Related to the previous comment: If attentional shifts on fingers can activate S1 somatotopically, could this potentially explain the results? Perhaps the participants were attending to the fingers that were cued to be moved and this would have led to the observed activity patterns. I don't think the data of the current study allows the authors to tease apart these potential contributions. It is likely that both processes contribute simultaneously.
We agree that our results could also be explained by attentional shifts on the fingers. It is very likely that, during planning, participants were specifically focusing on the cued finger. However, as the reviewer points out, our current dataset cannot distinguish between planning and attention as voluntary planning requires attention. We expanded the discussion section to include this possibility.
- The authors repeatedly interpret the absences of significant differences as indicating that the tested entities are the same. This cannot be concluded based on results of frequentist statistical testing. If the authors would like to make such claims, then they I think they should include Bayesian analysis to investigate the level of support for the null hypothesis.
We have now clarified the parts in the manuscript that sounded as if we were interpreting the absence of significant difference (null results) as significant absence of differences (equivalence).
-

Evaluation Summary:
The authors studied the neural correlates of planning and execution of single finger presses in a 7T fMRI study focusing on primary somatosensory (S1) and motor (M1) cortices. BOLD patterns of activation/deactivation and finger-specific pattern discriminability indicate that M1 and S1 are involved not only during execution, but also during planning of single finger presses. These results contribute to a developing story that the role of primary somatosensory cortex goes beyond pure processing of tactile information and will be of interest for researchers in the field of motor control and of systems neuroscience.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer …
Evaluation Summary:
The authors studied the neural correlates of planning and execution of single finger presses in a 7T fMRI study focusing on primary somatosensory (S1) and motor (M1) cortices. BOLD patterns of activation/deactivation and finger-specific pattern discriminability indicate that M1 and S1 are involved not only during execution, but also during planning of single finger presses. These results contribute to a developing story that the role of primary somatosensory cortex goes beyond pure processing of tactile information and will be of interest for researchers in the field of motor control and of systems neuroscience.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
This is a very important study for the field, as the involvement of S1 in motor planning has never been described. The paradigm is very elegant, the methods are rigorous and the manuscript is clearly written. However, there are some concerns about the interpretation of the data that could be addressed.
• The authors claim that planning and execution patterns are scaled version of each other, and that overt movement during planning is prevented by global deactivation. This is an interesting perspective, however the presented data are not fully convincing to support this claim:
(1) the PCM analysis shows that correlation models ranging from 0.4 to 1 perform similarly to the best correlation model. This correlation range is wide and suggests that the correspondence between execution/planning patterns is only …
Reviewer #1 (Public Review):
This is a very important study for the field, as the involvement of S1 in motor planning has never been described. The paradigm is very elegant, the methods are rigorous and the manuscript is clearly written. However, there are some concerns about the interpretation of the data that could be addressed.
• The authors claim that planning and execution patterns are scaled version of each other, and that overt movement during planning is prevented by global deactivation. This is an interesting perspective, however the presented data are not fully convincing to support this claim:
(1) the PCM analysis shows that correlation models ranging from 0.4 to 1 perform similarly to the best correlation model. This correlation range is wide and suggests that the correspondence between execution/planning patterns is only partial.
(2) in Fig.4 A-B, the distance between execution/planning patterns is much larger than the distance between fingers. How can such a big difference be explained if planning/execution correspond to scaled versions of the same finger-specific patterns? If the scaling is causing this difference, then different normalization steps of the patterns should have very specific effects on the observed results: 1) removing the mean value for each voxel (separately for execution and planning conditions) should nullify the scaling and the planning/execution patterns should perfectly align in a finger-specific way; 2) removing the mean pattern (separately for each finger conditions) should effectively disturb the finger-specific alignment shown in Fig.4C.
These analyses would corroborate the authors' conclusion.• A conceptual concern is related to the task used by the authors. During the planning phase, as a baseline task, participants are asked to maintain a low and constant force for all the fingers. This condition is not trivial and can even be considered a motor task itself. Therefore, the planning/execution of the baseline task might interfere with the planning/execution of the finger press task. Even more controversial, the design of the motor task might be capturing transitions between different motor tasks (force on all finger towards single-finger press) rather than pure planning/execution of a single task. The authors claim that the baseline task was used to control for involuntary movements, however, EMG recordings could have similarly controlled for this aspect, without any confounds.
• In Fig.2F, the authors show no-planning related information in high-order areas (PMd, aSPL), while such information is found in M1 and S1. This null result from premotor and parietal areas is rather surprising, considering current literature, largely cited by the authors, pointing to high-order motor or parietal areas involved in action planning.
-

Reviewer #2 (Public Review):
The present investigation aimed at exploring the role of primary somatosensory cortex (S1) in the representation of single finger movements during motor planning and the relationship of this representation with the one present during movement execution.
The authors conducted a high-field (7 Tesla) fMRI study focusing their analysis on the contralateral S1 and on the primary motor cortex (M1). Participants had to perform a delayed execution task, in which they were instructed to perform specific finger movements after a variable delay. After the delay, a go/no-go cue would instruct the participant either to perform or not perform the cued movement.
Univariate analysis showed a widespread deactivation within M1 and S1 during planning. Nevertheless, multivariate pattern analysis (MVPA) showed that information …
Reviewer #2 (Public Review):
The present investigation aimed at exploring the role of primary somatosensory cortex (S1) in the representation of single finger movements during motor planning and the relationship of this representation with the one present during movement execution.
The authors conducted a high-field (7 Tesla) fMRI study focusing their analysis on the contralateral S1 and on the primary motor cortex (M1). Participants had to perform a delayed execution task, in which they were instructed to perform specific finger movements after a variable delay. After the delay, a go/no-go cue would instruct the participant either to perform or not perform the cued movement.
Univariate analysis showed a widespread deactivation within M1 and S1 during planning. Nevertheless, multivariate pattern analysis (MVPA) showed that information about the upcoming finger movements was present both in M1 and S1 during the planning phase. The informative patterns of upcoming finger movements during the preparatory delay were highly correlated to the representation of finger movements during the execution phase of the task.
A control analysis excluded the possibility that the main results of the study might be caused by subtle differences in the forces exerted by the fingers during the planning phase. Indeed, the applied forces were comparable across fingers and they didn't predict neural activation.
The interpretations of these findings suggested a possible role of preparatory signals in S1 which might be to contribute to motor control either through predicting upcoming changes in sensory processing or through a direct effect on the spinal cord.
The present investigation is well-conducted and the analyses are solid and clear. The authors adopted a controlled experimental paradigm and design. In details, the authors adopted a delayed paradigm to dissociate the planning from the execution phase of the task and conducted the analysis only on the no-go trials avoiding any possible contamination with motor execution activity. Moreover, they conducted a further analysis to exclude that preparatory state of the participants in terms of the force exerted on the response apparatus. All these specific choices in how to implement the present study underline the expertise in the field of the authors.
With respect to the general aim of the study, I think the authors showed convincing evidence that S1 represents specific information about upcoming finger movements. Moreover, the additional control analyses further clarified the specific role of the S1, excluding possible confounding factors which might provide alternative interpretations to the present findings. The conclusions drawn in the manuscript are clearly supported by the results and in line with recent findings on the contribution of S1 to motor planning. To conclude, the present work provided novel insights on the contribution of the somatosensory cortex during motor control by adopting state-of-the-art analytical approaches.
-

Reviewer #3 (Public Review):
I found the manuscript to be well written and the study very interesting. There are, however, some analytical concerns that in part arise because of a lack of clarity in describing the analyses.
Some details regarding the methods used and results in the figures were missing or difficult to understand based on the brief description in the Methods section or figure legend.
I think the manuscript would benefit from a more balanced description on the role of S1. As the authors state, S1 is traditionally thought to process afferent tactile and proprioceptive input. However, in the past years, S1 has been shown to be somatopically activated during touch observation, attempted movements in the absence of afferent tactile inputs, and through attentional shifts (Kikkert et al., 2021; Kuehn et al., 2014; Puckett et …
Reviewer #3 (Public Review):
I found the manuscript to be well written and the study very interesting. There are, however, some analytical concerns that in part arise because of a lack of clarity in describing the analyses.
Some details regarding the methods used and results in the figures were missing or difficult to understand based on the brief description in the Methods section or figure legend.
I think the manuscript would benefit from a more balanced description on the role of S1. As the authors state, S1 is traditionally thought to process afferent tactile and proprioceptive input. However, in the past years, S1 has been shown to be somatopically activated during touch observation, attempted movements in the absence of afferent tactile inputs, and through attentional shifts (Kikkert et al., 2021; Kuehn et al., 2014; Puckett et al., 2017; Wesselink et al., 2019). Furthermore, S1 is heavily interconnected with M1, so perhaps if such activity patterns are present in M1, they could also be expected in S1?
Related to the previous comment: If attentional shifts on fingers can activate S1 somatotopically, could this potentially explain the results? Perhaps the participants were attending to the fingers that were cued to be moved and this would have led to the observed activity patterns. I don't think the data of the current study allows the authors to tease apart these potential contributions. It is likely that both processes contribute simultaneously.
The authors repeatedly interpret the absences of significant differences as indicating that the tested entities are the same. This cannot be concluded based on results of frequentist statistical testing. If the authors would like to make such claims, then they I think they should include Bayesian analysis to investigate the level of support for the null hypothesis.
-