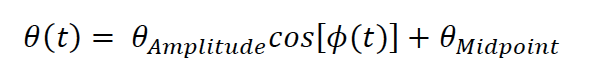Effects of arousal and movement on secondary somatosensory and visual thalamus
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The authors show that secondary thalamic region POm provides information on slow as opposed to rapid changes in whisker angular position; these appear to be secondary to changes in the animal's behavioral state. The authors find similar state dependent activity in LP, a higher level visual thalamic nucleus. This is a timely study in that many labs have observed state-dependent activity throughout the cortex and thalamus, but the mechanisms of this activity are incompletely understood. This study brings us closer to revealing the source of this signal by ruling out some of the likely candidates, such as reafferent signals and cortical feedback.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Neocortical sensory areas have associated primary and secondary thalamic nuclei. While primary nuclei transmit sensory information to cortex, secondary nuclei remain poorly understood. We recorded juxtasomally from secondary somatosensory (POm) and visual (LP) nuclei of awake mice while tracking whisking and pupil size. POm activity correlated with whisking, but not precise whisker kinematics. This coarse movement modulation persisted after facial paralysis and thus was not due to sensory reafference. This phenomenon also continued during optogenetic silencing of somatosensory and motor cortex and after lesion of superior colliculus, ruling out a motor efference copy mechanism. Whisking and pupil dilation were strongly correlated, possibly reflecting arousal. Indeed LP, which is not part of the whisker system, tracked whisking equally well, further indicating that POm activity does not encode whisker movement per se. The semblance of movement-related activity is likely instead a global effect of arousal on both nuclei. We conclude that secondary thalamus monitors behavioral state, rather than movement, and may exist to alter cortical activity accordingly.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
The authors make juxtacellular recordings on awake mice, which should yield clear responses of actions potentials, and employ a number of manipulations to silence pathways. They also record from a "non"-whisker secondary thalamic region, LP, as a null hypothesis to establish if certain effects are related to "behavior" - read arousal or saliency". I have no major qualms.
In light of Petersen's paper (Cell Reports 2014) on cholinergic effects on spike rates in primary whisker somatosensory cortex, I can imagine that the authors considered measuring from cholinergic neurons in nucleus basalis during whisking. I'll assume that this is easier said than done. As such, the current manuscript passes my threshold for publication modulo issues raised below that are related to anatomy.
The …
Author Response:
Reviewer #1 (Public Review):
The authors make juxtacellular recordings on awake mice, which should yield clear responses of actions potentials, and employ a number of manipulations to silence pathways. They also record from a "non"-whisker secondary thalamic region, LP, as a null hypothesis to establish if certain effects are related to "behavior" - read arousal or saliency". I have no major qualms.
In light of Petersen's paper (Cell Reports 2014) on cholinergic effects on spike rates in primary whisker somatosensory cortex, I can imagine that the authors considered measuring from cholinergic neurons in nucleus basalis during whisking. I'll assume that this is easier said than done. As such, the current manuscript passes my threshold for publication modulo issues raised below that are related to anatomy.
The cholinergic experiments are an interesting idea. However, inactivation of S1 did not change the relationship between POm and whisking, suggesting that cholinergic modulation of S1 and thereby corticothalamic output are not the key mechanism. It is conceivable that acetylcholine modulates POm directly, but the critical experiments would involve extensive manipulations of POm (a whole additional study). Nevertheless, we have added a reference to Eggermann & Petersen and discussed this issue further in the revision.
I provide a figure-by-figure critique:
(1) Recent work from Deschênes et al (Neuron 2016) points to a description of whisking in terms of Angle = Set-point_angle - Whisking-amplitude [1 + cosine(Phase - Phase_0)], where Phase is a rapidly varying, typically rhythmic function of time. Why not use this notation as opposed to yet another descriptive statistic and report the kinetics as the time averaged parameters , i.e., the most forward position, and ,Whisking-amplitude>, i.e., the half-amplitude of the average whisk?
We are not entirely sure what the reviewer means by “another descriptive statistic” as we do not introduce new approaches for analyzing whisking in this paper. (Perhaps the reviewer refers to “median angle”, which is an average of all the whisker positions on a single frame. We use this measurement because our videos contain the entire whisker field rather than just a single whisker as in our other studies, e.g. Hong et al 2018, Rodgers et al 2021). We based our parameterization of median angle on two publications: Hill et al (2011 Neuron) and Moore et al (2015 PLoS Biology). Moore et al describes whisking as a function of phase, amplitude, and midpoint:
where 𝜃(t) is the median whisker angle at time 𝑡 , 𝜙 is the phase as computed by the Hilbert transform of the filtered whisker angle, 𝜃^Amplitude is the difference between the most protracted and retracted whisker positions over a single cycle, and 𝜃^midpoint is the central angle of a single whisk cycle. As we understand the reviewer, we are using the formulation they describe. We are happy to consider alternate formulations if we are missing something.
A critical issue is to confirm where the recording were made. This the authors should supply at least a typical record of anatomy from their POm as well as VPM and LP recording. The beauty of the juxtacellular technique is that neurons can be labeling after the recording
We used the juxtacellular recording technique for its superior recording quality. We did not label individual cells after recording because we recorded multiple cells per animal over several days. The number of cells would complicate matching of filled cells to recorded physiological data, and biotin filling is not stable over multiple days (beyond 36 hours). Instead, as described in the original manuscript, we tracked the relative locations of all inserted pipettes and labeled the final track with DiI. Cells were roughly localized along the tracks using relative microdrive depths. Due to the morphological homogeneity of thalamic neurons, filling individual cells would not be more informative than labelling the recording site with DiI. New Figure 1 – figure supplement 1 includes representative histology images from our recordings in POm, VPM, LP, and M1.
(2) Did the authors make sure that the mystacial pad is not moving by imaging the pad as opposed to just the shaft of the whiskers? The top view in Figure 1A makes this hard to check.
To address this concern, we provide new data, in which both the cut and uncut sides of the face of mice were imaged. We measured the movement of the mystacial pads as motion energy – the mean absolute difference in pixel values across video frames. The motor nerve surgery almost completely abolished movement of the mystacial pad. A new figure panel (Figure 2B) demonstrates the movement of the normal and paralyzed mystacial pads.
Further, did the authors perform post-hoc anatomy to insure that both the ramus buccolabialis inferior and ramus buccolabialis superior muscles were cut? This is critical; it is also easy to leave the maxillolabialis (external retractor) innervated if the cut is too far rostral.
We did not attempt to cut muscles. We only cut the motor nerve. We did not examine the face post mortem, as it was obvious that both whisker and mystacial pad movement were absent (as in new Figure 2B).
(3/4) As relevant background, the text should note that whisker primary motor cortex maintains a copy of the envelope of the whisking, i.e., an ill-defined summation of set-point and amplitudes, even if the sensory input (Ahrens & Kleinfeld J Neurophysiol 2004) or motor output (Fee et al. J Neurophysiol 1997) in the periphery are cut.
The Results text now cites these papers as motivation for the experiments of Figure 3.
(6/7) Same comments in (1) in whisking parameters and anatomy.
As we discussed in (1), we are using the conventional parameterization of others. Histological examples are now included in Figure 1 – figure supplement 1.
Reviewer #3 (Public Review):
Previous studies in urethane-anesthetized rats (PMID 16605304) proposed that POm cells code whisker movements. This was observed using "artificial whisking" procedures (stimulating the motor nerve to produce a whisking-like movement). It has been clear for some time now that there are substantial (obvious) differences between this procedure and natural whisking. In addition, under urethane-anesthesia animals are in a sleep-like state that is very dissimilar to waking (although some work has tested the effect of network state on artificial whisking responses in both primary thalamus and cortex; see 25505118). In the present study, the authors measured activity in POm cells during whisking in awake (head-fixed) mice to determine if they code whisking movement. However, this seems to have already been done previously. For instance, Moore et al (2015; 26393890) found that coding of whisking in the ascending paralemniscal pathway, including POm, is "relatively poor" (as stated in the abstract), which is the same conclusion reached in the present study. The authors should clarify the main differences observed in whisking coding between their study and previous work.
The authors then focused on the idea that POm codes behavioral state. However, many studies have previously determined that state has a great impact on thalamocortical dynamics; thalamic cells are very sensitive to state including cells in primary whisker thalamic nuclei, such as VPM, and these effects can be produced by neuromodulators (see work by Castro-Alamancos' group, for example, 16306412). There is nothing special about VPM in this regard; other thalamic sensory nuclei are also sensitive to behavioral state and neuromodulators. Therefore, the observation that POm and LP cells are sensitive to state is unsurprising. It is also known that these thalamic state changes have a great impact on the state of the cortex (see 20053845), which seems very relevant to the main conclusion. The POm has to be doing something different than coding behavioral state since most thalamic nuclei do this. The study did not identify the role of POm, which certainly has to be different from LP (otherwise, why would these nuclei be differentiated?). POm is unlikely to be specialized for monitoring state since this is done by most of the thalamus -including VPM, which projects to the same cortical region. Thus, while it is interesting that most of the whisker-related activity in POm is state-dependent, the study does not clarify the role of POm.
We have added the references we did not already include to our text and improved our discussion.
Prior studies (such as Moore et al 2015 and Urbain et al 2015) have previously characterized the encoding of whisker motion in POm. Indeed, we note the consistency between our results and such studies in both the introduction and conclusion. Here we expand upon prior studies to directly test two prominent hypotheses about the role of the paralemniscal pathway: that it encodes sensory reafference, and that it inherits a motor efference copy from cortical and subcortical regions. We present the impact of several manipulations of the vibrissal system (facial paralysis, cortical silencing, and lesion of superior colliculus) on thalamic activity that, to our knowledge, have not been previously reported. Moreover, we leveraged a novel comparison of POm and LP to test whether movement‐correlations of POm reflected true motor modulation or rather state dependency. We have provided evidence that the coupling of POm activity to whisking reflects state rather than motor signals. We never suggested that POm is a unique monitor of behavioral state. We suggest instead that secondary thalamic nuclei may be state‐modulated and have specific impacts on response gain and plasticity in their respective cortical areas. While our work is consistent with previous studies, we believe these results are novel extensions of past work.
The main strength of the study is that it was performed in awake mice with behavioral state monitoring, which contributes to the current understanding of active whisking coding in the complex network of the vibrissa system.
In our opinion, the main strength of our study is its multiple manipulations to test the sources of modulation and the leveraging of a POm‐LP comparison. We have revised the text to reinforce these points.
-

Evaluation Summary:
The authors show that secondary thalamic region POm provides information on slow as opposed to rapid changes in whisker angular position; these appear to be secondary to changes in the animal's behavioral state. The authors find similar state dependent activity in LP, a higher level visual thalamic nucleus. This is a timely study in that many labs have observed state-dependent activity throughout the cortex and thalamus, but the mechanisms of this activity are incompletely understood. This study brings us closer to revealing the source of this signal by ruling out some of the likely candidates, such as reafferent signals and cortical feedback.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the …
Evaluation Summary:
The authors show that secondary thalamic region POm provides information on slow as opposed to rapid changes in whisker angular position; these appear to be secondary to changes in the animal's behavioral state. The authors find similar state dependent activity in LP, a higher level visual thalamic nucleus. This is a timely study in that many labs have observed state-dependent activity throughout the cortex and thalamus, but the mechanisms of this activity are incompletely understood. This study brings us closer to revealing the source of this signal by ruling out some of the likely candidates, such as reafferent signals and cortical feedback.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
The authors make juxtacellular recordings on awake mice, which should yield clear responses of actions potentials, and employ a number of manipulations to silence pathways. They also record from a "non"-whisker secondary thalamic region, LP, as a null hypothesis to establish if certain effects are related to "behavior" - read arousal or saliency". I have no major qualms.
In light of Petersen's paper (Cell Reports 2014) on cholinergic effects on spike rates in primary whisker somatosensory cortex, I can imagine that the authors considered measuring from cholinergic neurons in nucleus basalis during whisking. I'll assume that this is easier said than done. As such, the current manuscript passes my threshold for publication modulo issues raised below that are related to anatomy.
I provide a figure-by-figure …
Reviewer #1 (Public Review):
The authors make juxtacellular recordings on awake mice, which should yield clear responses of actions potentials, and employ a number of manipulations to silence pathways. They also record from a "non"-whisker secondary thalamic region, LP, as a null hypothesis to establish if certain effects are related to "behavior" - read arousal or saliency". I have no major qualms.
In light of Petersen's paper (Cell Reports 2014) on cholinergic effects on spike rates in primary whisker somatosensory cortex, I can imagine that the authors considered measuring from cholinergic neurons in nucleus basalis during whisking. I'll assume that this is easier said than done. As such, the current manuscript passes my threshold for publication modulo issues raised below that are related to anatomy.
I provide a figure-by-figure critique:
(1) Recent work from Deschênes et al (Neuron 2016) points to a description of whisking in terms of Angle = Set-point_angle - Whisking-amplitude [1 + cosine(Phase - Phase_0)], where Phase is a rapidly varying, typically rhythmic function of time. Why not use this notation as opposed to yet another descriptive statistic and report the kinetics as the time averaged parameters <Set-point_angle>, i.e., the most forward position, and ,Whisking-amplitude>, i.e., the half-amplitude of the average whisk?
A critical issue is to confirm where the recording were made. This the authors should supply at least a typical record of anatomy from their POm as well as VPM and LP recording. The beauty of the juxtacellular technique is that neurons can be labeling after the recording(2) Did the authors make sure that the mystacial pad is not moving by imaging the pad as opposed to just the shaft of the whiskers? The top view in Figure 1A makes this hard to check. Further, did the authors perform post-hoc anatomy to insure that both the ramus buccolabialis inferior and ramus buccolabialis superior muscles were cut? This is critical; it is also easy to leave the maxillolabialis (external retractor) innervated if the cut is too far rostral.
(3/4) As relevant background, the text should note that whisker primary motor cortex maintains a copy of the envelope of the whisking, i.e., an ill-defined summation of set-point and amplitudes, even if the sensory input (Ahrens & Kleinfeld J Neurophysiol 2004) or motor output (Fee et al. J Neurophysiol 1997) in the periphery are cut.
(6/7) Same comments in (1) in whisking parameters and anatomy.
-

Reviewer #2 (Public Review):
The authors are attempting to reveal the nature and source of state-dependent activity in a somatosensory nucleus of the mouse. They observe that PoM exhibits activity related to the slow components of whisker movements. Block of reafferent singles from the face, by nerve-cut induced paralysis, optogenetic inhibition of somatosensory cortex, or electrolytic lesion of the superior colliculi are all unable to block the whisker-related movement signal in PoM. The authors find similar state dependent activity in the LP nucleus, which is a higher level visual thalamic nucleus. They suggest that the activity is more related to arousal than movement, per se.
This is a timely study in that many labs have observed state-dependent activity throughout the cortex and thalamus, but the mechanisms of this activity are …
Reviewer #2 (Public Review):
The authors are attempting to reveal the nature and source of state-dependent activity in a somatosensory nucleus of the mouse. They observe that PoM exhibits activity related to the slow components of whisker movements. Block of reafferent singles from the face, by nerve-cut induced paralysis, optogenetic inhibition of somatosensory cortex, or electrolytic lesion of the superior colliculi are all unable to block the whisker-related movement signal in PoM. The authors find similar state dependent activity in the LP nucleus, which is a higher level visual thalamic nucleus. They suggest that the activity is more related to arousal than movement, per se.
This is a timely study in that many labs have observed state-dependent activity throughout the cortex and thalamus, but the mechanisms of this activity are incompletely understood. Since this activity accounts for a large fraction of the activity of the forebrain in awake mice, it is a priority to understand the mechanisms of its generation. This study brings us closer to revealing the source of this signal.
Unfortunately, the authors do not discover the source of the arousal/movement signal - but give evidence against some of the more popular/likely candidates.
-

Reviewer #3 (Public Review):
Previous studies in urethane-anesthetized rats (PMID 16605304) proposed that POm cells code whisker movements. This was observed using "artificial whisking" procedures (stimulating the motor nerve to produce a whisking-like movement). It has been clear for some time now that there are substantial (obvious) differences between this procedure and natural whisking. In addition, under urethane-anesthesia animals are in a sleep-like state that is very dissimilar to waking (although some work has tested the effect of network state on artificial whisking responses in both primary thalamus and cortex; see 25505118). In the present study, the authors measured activity in POm cells during whisking in awake (head-fixed) mice to determine if they code whisking movement. However, this seems to have already been done …
Reviewer #3 (Public Review):
Previous studies in urethane-anesthetized rats (PMID 16605304) proposed that POm cells code whisker movements. This was observed using "artificial whisking" procedures (stimulating the motor nerve to produce a whisking-like movement). It has been clear for some time now that there are substantial (obvious) differences between this procedure and natural whisking. In addition, under urethane-anesthesia animals are in a sleep-like state that is very dissimilar to waking (although some work has tested the effect of network state on artificial whisking responses in both primary thalamus and cortex; see 25505118). In the present study, the authors measured activity in POm cells during whisking in awake (head-fixed) mice to determine if they code whisking movement. However, this seems to have already been done previously. For instance, Moore et al (2015; 26393890) found that coding of whisking in the ascending paralemniscal pathway, including POm, is "relatively poor" (as stated in the abstract), which is the same conclusion reached in the present study. The authors should clarify the main differences observed in whisking coding between their study and previous work.
The authors then focused on the idea that POm codes behavioral state. However, many studies have previously determined that state has a great impact on thalamocortical dynamics; thalamic cells are very sensitive to state including cells in primary whisker thalamic nuclei, such as VPM, and these effects can be produced by neuromodulators (see work by Castro-Alamancos' group, for example, 16306412). There is nothing special about VPM in this regard; other thalamic sensory nuclei are also sensitive to behavioral state and neuromodulators. Therefore, the observation that POm and LP cells are sensitive to state is unsurprising. It is also known that these thalamic state changes have a great impact on the state of the cortex (see 20053845), which seems very relevant to the main conclusion. The POm has to be doing something different than coding behavioral state since most thalamic nuclei do this. The study did not identify the role of POm, which certainly has to be different from LP (otherwise, why would these nuclei be differentiated?). POm is unlikely to be specialized for monitoring state since this is done by most of the thalamus -including VPM, which projects to the same cortical region. Thus, while it is interesting that most of the whisker-related activity in POm is state-dependent, the study does not clarify the role of POm.
The main strength of the study is that it was performed in awake mice with behavioral state monitoring, which contributes to the current understanding of active whisking coding in the complex network of the vibrissa system.
-