Ecological analysis of Pavlovian fear conditioning in rats
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This paper is of potential interest to a broad audience of neuroscientists. By concluding that fear conditioning does not occur in a semi-naturalistic experimental setup, the study implies a major adjustment in our current understanding of Pavlovian fear conditioning and associative learning. However, additional controls and data analyses are required to validate the authors' conclusions.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Pavlovian fear conditioning, which offers the advantage of simplicity in both the control of conditional and unconditional stimuli (CS, US) presentation and the analysis of specific conditional and unconditional responses (CR, UR) in a controlled laboratory setting, has been the standard model in basic and translational fear research. Despite 100 years of experiments, the utility of fear conditioning has not been trans-situationally validated in real-life contexts. We thus investigated whether fear conditioning readily occurs and guides the animal’s future behavior in an ecologically-relevant environment. To do so, Long-Evans rats foraging for food in an open arena were presented with a tone CS paired with electric shock US to their dorsal neck/body that instinctively elicited escape UR to the safe nest. On subsequent test days, the tone-shock paired animals failed to exhibit fear CR to the CS. In contrast, animals that encountered a realistic agent of danger (a looming artificial owl) paired with a shock, simulating a plausible predatory strike, instantly fled to the nest when presented with a tone for the first time. These results highlight the possibility of a nonassociative, rather than standard associative, fear process providing survival function in life-threatening situations that animals are likely to encounter in nature.
Article activity feed
-
-

Author Response:
Reviewer #1 (Public Review):
This manuscript describes a series of behavioral experiments in which foraging rats are subjected to a novel fear conditioning paradigm. Different groups of animals receive a shock to the dorsal surface of the body paired with either tone, an artificial owl driven forward with pneumatic pressure, or a tone/owl combination. An additional control condition pairs tone with owl alone (ie no shock is delivered). In a subsequent test, only owl+shock and tone/owl+shock animals show increased latency to forage and a withdrawal response to tone (even though owl-shock rats do not experience tone during conditioning). The authors conclude that this tone response is due to sensitization and that fear conditioning does not occur in their experimental setup.
This approach is intriguing and the issues …
Author Response:
Reviewer #1 (Public Review):
This manuscript describes a series of behavioral experiments in which foraging rats are subjected to a novel fear conditioning paradigm. Different groups of animals receive a shock to the dorsal surface of the body paired with either tone, an artificial owl driven forward with pneumatic pressure, or a tone/owl combination. An additional control condition pairs tone with owl alone (ie no shock is delivered). In a subsequent test, only owl+shock and tone/owl+shock animals show increased latency to forage and a withdrawal response to tone (even though owl-shock rats do not experience tone during conditioning). The authors conclude that this tone response is due to sensitization and that fear conditioning does not occur in their experimental setup.
This approach is intriguing and the issues raised by the manuscript are extremely important for the field to consider. However, there are many ways to interpret the results as they stand. One issue of primary importance is whether it can indeed be claimed that conditioning did not readily occur in the tone+shock group. The lack of a particular behavioral conditioned reaction does not equate to an absence of conditioning. It is possible that unseen (i.e. physiological) measures of conditioning, many of which were once standard DVs in the fear conditioning literature, are present in the tone+shock group. This possibility pushes against the claim made in the title and elsewhere. These claims should be softened.
We agree with the reviewer and now acknowledge the following caveat in the discussion (pg. 10): “…although neither the tone-shock group nor the tone-owl group showed overt manifestations of fear conditioning (as measured by fleeing or freezing) to the tone that prevented a successful procurement of food, the possibility of physiological (e.g., cardiovascular, respiratory) changes associated with tone-induced fear (Steimer, 2002) cannot be excluded in these animals…”
Because systemic, group-level retreat CRs are not noted in the tone+shock condition, it would indeed be important to establish if there are any experimental circumstances in which tone paired with a US applied to the dorsal surface of the body can produce consistent reactions (e.g. freezing) to tone alone. Though it may seem likely that tone + dorsal shock would indeed produce freezing in a different setting, this result should not be taken for granted - we've known since the 'noisy water' experiment (Garcia & Koelling, 1966) that not every CS pairs with every US and that association can indeed be selective. A positive control would be clarifying. If the authors could demonstrate that tone+dorsal shock produces freezing to tone in a commonly used fear conditioning setup (ie standard cubicle chamber) then the lack of a retreat CR in their naturalistic paradigm would gain added meaning.
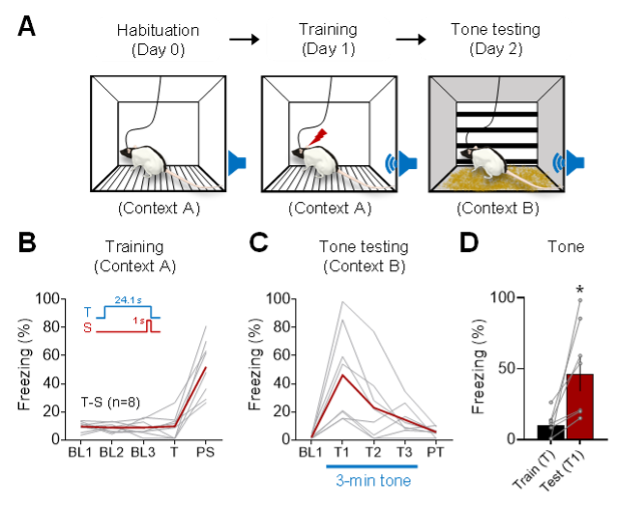
This is an excellent suggestion. As recommended, we performed a positive control experiment where naïve rats that underwent the same subcutaneous wire implant surgery were placed in a standard experimental chamber and presented with a delayed tone-shock pairing (same tone frequency/intensity and shock intensity/duration; the 24.1 s CS duration was based on the mean CS duration of tone-shock animals in the naturalistic fear conditioning experiment). As can be seen in Author response image 1 (Figure 4 in the revised manuscript) below, these animals exhibited reliable postshock freezing in a conditioning chamber (fear conditioning day 1) and tone CS-evoked freezing in a novel chamber (tone testing day 2), indicating that our original finding (i.e., no evidence of auditory and contextual fear conditioning in an ecologically-relevant environment) is unlikely due to a dorsal neck/body shock US per se.
Author response image 1. Auditory fear conditioning in a standard experimental chamber. (A) Illustrations of a rat implanted with wires subcutaneously in the dorsal neck/body region undergoing successive days of habituation (10 min tethered, conditioning chamber), training (a single tone CS-shock US pairing), and tone testing (context shift). (B) Mean (crimson line) and individual (gray lines) percent freezing data from 8 rats (4 females, 4 males) during training in context A: 3 min baseline (BL1, BL2, BL3); 23.1 s epoch of tone (T) excluding 1 s overlap with shock (S); 1 min postshock (PS). (C) Mean and individual percent freezing data during tone testing in context B: 1 min baseline (BL1); 3 min tone (T1, T2, T3); 1 min post-tone (PT). (D) Mean + SEM (bar) and individual (dots) percent freezing to tone CS before (Train, T) and after (Test, T1) undergoing auditory fear conditioning (paired t-test; t(7) = -3.163, p = 0.016). * p < 0.05
The altered withdrawal trajectory seen in owl+shock and tone/owl+shock groups occurs in neither the tone+shock nor the tone+owl group, introducing the possibility that it results from the specific pairing of owl and shock. Put differently - this response may indeed by an associative CR. Do altered withdrawal angles persist if animals that receive owl+shock are exposed to owl again the next day? Do manipulations of the owl and shock that diminish fear conditioning (e.g. unpairing of owl and shock stimuli) eliminate deflected withdrawal angles when the subject is exposed to owl alone? If so, it would cut against the interpretation that fear conditioning does not occur in the setup described here, and would instead demonstrate that it is indeed central to predatory defense. This interpretation is compatible with the effect of hippocampal lesion on freezing evoked by a live predator. Destruction of the rat hippocampus diminishes cat-evoked freezing - this is thought to occur because the rapid association of the cat's various features with threatening action is not formed by the rat (Fanselow, 2000, 2018). Even though this interpretation of the results differs from the authors', it in no way diminishes the interest of this work. This paradigm may indeed be a novel means by which to study rapidly acquired associations with ethological relevance. Follow-up experiments of the type described above are necessary to disambiguate opposing views of the current dataset.
Whether “altered withdrawal angles persist if animals that receive owl+shock [a US-US pairing] are exposed to owl again the next day” is an interesting question, as it is conceivable that the owl US (Zambetti et al., 2019, iScience) can function as a CS to evoke anticipatory characteristic of the conditioned fear. This possibility is now mentioned as a caveat (pg. 10): “…the erratic escape trajectory behavior exhibited by owl-shock and tone/owl-shock animals may be indicative of rapid associative processes at work (Fanselow 2018). For example, the immediate-shock (and delayed shock-context shift) deficit in freezing (e.g., Fanselow 1986; Landeira-Fernandez et al., 2006) provides compelling evidence that postshock freezing is not a UR but rather a CR to the contextual representation CS that rapidly became associated with the footshock US. In a similar vein then the erratic escape CR topography in owl-shock and tone/owl-shock animals might represent a shift in ‘functional CR topography’ (Fanselow & Wassum 2016) resulting from the rapid association between some salient features of the owl and the dorsal neck/body shock. A rapid owl-shock association nevertheless cannot explain the owl-shock animals’ subsequent fleeing behavior to a novel tone (in the absence of owl), which likely reflects nonassociative fear.”
Reviewer #2 (Public Review):
This work is dealing with an interesting question whether a simple, one trial CS+US (Pavlovian) association occurs in a naturalistic environment. Pavlovian fear conditioning contains a repetition of a neutral sensory signal (tone, CS) which is paired with a mild US, usually foot-shock (<1 mA; thus, unpleasant rather than painful) and the CS+US association drives associative learning. In this paper, a single 2.5 mA electrical shock was paired with a novel 80 dB tone to monitor the occurrence of learning via measuring success rate and latency of foraging for food. Some animals experienced an owl-looming matched with the US, just before reaching the food. The authors placed hunger-motivated rats into a custom-built arena equipped with safe nest, gate, food zone as well as with a delivery of a self-controlled US (electrical shock in the neck muscle and/or owl-looming). The US was activated by the rats by approaching to the food. Thus, a conflicting situation was provoked where procuring the food is paired with an aversive conditioned signal. Four groups of rats were included in the experiments based on their conditioning types: tone+ shock, tone+ shock+ owl, shock+owl and tone+owl. Due to these conditioning procedures, none of the rat procured the food but fled to the nest. In contrast, in the retrieval phases (next two days), the tone-shock and tone-owl groups successfully procured the pellets but not the tone-shock-owl group during the conditioned tone presentation. Rats in the latter group fled to the nest upon tone presentation at the food zone. As the shock-owl animals (conditioned without tone) also fled to the nest triggered by (unfamiliar) tone presentation, their and the tone+shock+owl group's fled responses were assigned to be non-associative sensitization-like process. Furthermore, during the pre-tone trials, all groups showed similar behavior as in the tone test. These findings led the authors to conclude that classical Pavlovian fear conditioning may not present in an ecologically relevant environment.
The raised question is relevant for broad audience of neuroscience and behavioral scientist. However, as the used fear conditioning paradigm is not a common one, it is difficult to interpret the finding. It is based on a single pairing of an unfamiliar, salient tone with a very strong (traumatizing?) electrical shock, delivered directly into the neck muscle and an innate signal (owl looming). In addition, as the tone presentation was followed by many events (gate opening, presence of food, shock and/or owl-looming) in front of the animals, it is hard to image what sort of tone association could be formed at all.
We thank the reviewer for mentioning several important considerations. In regards to the shock amplitude used here, fear conditioning studies in rats have employed a wide range of numbers, durations and intensities of footshock; e.g., three footshocks: 1.0 mA/0.75-s and 4.0 mA/3-s (Fanselow 1984), 75 footshocks: 1 mA/2-s (Maren 1999; Zimmerman et al. 2007). Note also that 16-20 periorbital shocks (2.0 mA, 8 pulse train at 5 Hz) have been used in auditory fear conditioning in rats (Moita et al. 2003; Blair et al. 2005). Thus, it is unlikely that a single 2.5 mA dorsal neck/body shock (subcutaneous and not in the neck muscle) used in the present study is particularly traumatizing compared to higher intensity/longer duration (e.g., 4.0 mA/3-s) and far more numerous (e.g., 75) footshocks employed in fear conditioning studies.
The relationship between footshock intensity and fear conditioning also warrants further discussion. Sigmundi, Bouton, and Bolles (1980) examined conditioned freezing in rats to 15 footshocks of 0.5, 1.0 and 2.0 mA intensities (0.5-s duration) and found that “[tone] CS-evoked freezing increased with US intensity.” In contrast, Fanselow (1984) observed relatively higher contextual freezing in rats subjected to three bouts of 1.0 mA/0.75-s than 4 mA/3-s footshocks. Irrespective, the animals that received three 4 mA/3-s footshocks still exhibited robust freezing. Based on the positive control experimental results (see above), it is unlikely that the present study’s failure to observe conditioned fear is due to the use of 2.5 mA shock intensity.
As the animals in the present study underwent 5 baseline days of foraging (3 trials per day), they would have been habituated to the computer-controlled automated gate opening-closing and the presence of food by the time of tone-shock, tone-owl, owl-shock and tone-owl/shock events, making it unlikely that the tone would associate with the gate/food stimuli. In the employed delay conditioning configuration, the tone CS has greater temporal contiguity with the US (shock and/or owl) and the US is both novel and surprising relative to the other stimuli in the arena environment. Thus, it is more plausible that the tone CS would be associated with the intended US. In summary, we believe that if fear conditioning necessitates relatively sterile environmental settings in order to transpire, then fear conditioning would be implausible in the natural world filled with dynamic, complex stimuli.
One could also argue that if a hungry animal does not try to collect food after an unpleasant, even a painful experience, then, it normally dies soon (thus, that is not a 'natural' behavior). The tone+shock and tone+owl groups showed similar behavioral features throughout the entire experiments and may reconcile the natural events: although these rats had had negative experience before, were still approaching to food zone due their hunger. Because of their motivation for food, the authors concluded that no association was formed. Based on this single measure, is it right to do so?
In nature, prey animals adjust their foraging behavior to minimize danger (e.g., Stephens and Krebs 1986 Foraging Theory; Lima and Dill 1990 Can J Zool); thus, it is improbable that an aversive experience will lead to end of food seeking behavior leading to death. Indeed, Choi and Kim (2010 Proc Natl Acad Sci) employed a similar seminaturalistic environment (as the present study) and found that rats adjust their foraging behavior as a function of the predatory threat distance, consistent with the “predatory imminence” model (Fanselow and Lester 1988). Since only behavioral measures of fear were assessed (i.e., fleeing, latency to enter forage zone, pellet procurement), we now acknowledge a caveat in the discussion (see response to Reviewer 1’s comment 1). Note, however, that unlike the tone-shock paired animals that failed to flee to the tone CS and successfully procured the food pellet, the owl-shock animals exhibited robust fear behavior (promptly fled, ceasing foraging) to a novel tone.
Reviewer #3 (Public Review):
In this study, the authors aimed to test whether rats could be fear conditioned by pairing a subdermal electric shock to a tone, an owl-like approaching stimulus, or a combination of these in a naturalistic-like environment. The authors designed a task in which rats foraging for food were exposed to a tone paired to a shock, an owl-like stimulus, a combination of the owl and the shock, or paired the owl to a shock in a single trial. The authors indexed behaviors related to food approach after conditioning. The authors found that animals exposed to the owl-shock or the tone/owl-shock pairing displayed a higher latency to approach the food reward compared to animals that were presented with the tone-shock or the tone-owl pairing. These results suggest that pairing the owl with the shock was sufficient to induce inhibitory avoidance, whereas a single pairing of the tone-shock or the tone-owl was not. The authors concluded that standard fear conditioning does not readily occur in a naturalistic-like environment and that the inhibitory avoidance induced by the owl-shock pairing could be the result of increased sensitization rather than a fear association.
Strengths:
The manuscript is well-written, the behavioral assay is innovative, and the results are interesting. The inclusion of both males and females, and the behavioral sex comparison was commendable. The findings are timely and would be highly relevant to the field.
Weaknesses:
However, in its current state, this study does not provide convincing evidence to support their main claim that Pavlovian fear conditioning does not readily occur in naturalistic environments. The innovative task presented in this study is more akin to an inhibitory avoidance task rather than fear conditioning and should be reframed in such way.
The reviewer’s comment is theoretically important in translating laboratory studies of fear to real world situations. Because our animals were engaged in a purposive/goal-oriented foraging behavior, that is, the leaving of nest in search of food in an open space brought about tone-shock, tone-owl, owl-shock and tone/owl shock outcomes, one can make the case that this is in principle an inhibitory avoidance (instrumental fear conditioning) task rather than a Pavlovian fear conditioning task. A pertinent question then is whether procedurally ‘pure’ laboratory Pavlovian conditioning tasks (i.e., displacing animals from their home cage to an experimental chamber and presenting CS and US) are possible in real world settings where behaviors of animals and humans are largely purposive/goal-oriented (Tolman 1948 Psychol Rev). It is generally accepted that “Outside the laboratory, stimulus [Pavlovian] learning and response [Instrumental] learning are almost inseparable (Bouton 2007 Learning and Behavior, pg. 28).” The goal of our study was to investigate whether widely-employed auditory fear conditioning readily produces associative fear memory that guides future behavior in animals performing naturalistic foraging behavior, and insofar as presenting a salient tone CS followed by an aversive shock US, the present study has a Pavlovian fear component.
We thank the reviewer for raising this concern and have addressed the Pavlovian vs. Instrumental fear conditioning aspects of our study in the revised manuscript (pg. 10): “…there are obvious procedural differences between standard fear conditioning versus naturalistic fear conditioning. In the former paradigm, typically ad libitum fed animals are placed in an experimental chamber for a fixed time before receiving a CS-US pairing (irrespective of their ongoing behavior). Thus, the CS duration and ISI are constant across subjects. In our study, hunger-motivated rats searching for food must navigate to a fixed location in a large arena before experiencing a CS-US pairing (instrumental- or response-contingent). Because animals approach the US trigger zone at different latencies, the CS duration and ISI are variable across subjects.”
References
Bernstein, I. L., Vitiello, M. V., & Sigmundi, R. A. (1980). Effects of interference stimuli on the acquisition of learned aversions to foods in the rat. J Comp Physiol Psychol, 94(5), 921-931. doi:10.1037/h0077807
Blair, H. T., Huynh, V. K., Vaz, V. T., Van, J., Patel, R. R., Hiteshi, A. K., . . . Tarpley, J. W. (2005). Unilateral storage of fear memories by the amygdala. J Neurosci, 25(16), 4198-4205. doi:10.1523/JNEUROSCI.0674-05.2005
Bouton, M. E. (2007). Learning and Behavior: Sinauer Associates
Choi, J. S., & Kim, J. J. (2010). Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc Natl Acad Sci U S A, 107(50), 21773-21777. doi:10.1073/pnas.1010079108
Fanselow, M. S. (1984). Shock-induced analgesia on the formalin test: effects of shock severity, naloxone, hypophysectomy, and associative variables. Behav Neurosci, 98(1), 79-95. doi:10.1037//0735-7044.98.1.79
Fanselow, M. S. (1986). Associative Vs Topographical Accounts of the Immediate Shock Freezing Deficit in Rats - Implications for the Response Selection-Rules Governing Species-Specific Defensive Reactions. Learning and Motivation, 17(1), 16-39. doi:Doi 10.1016/0023-9690(86)90018-4
Fanselow, M. S. (2018). The Role of Learning in Threat Imminence and Defensive Behaviors. Curr Opin Behav Sci, 24, 44-49. doi:10.1016/j.cobeha.2018.03.003
Fanselow, M. S., & Lester, L. S. (1988). A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior: Lawrence Erlbaum Associates Inc.
Fanselow, M. S., & Wassum, K. M. (2016). The Origins and Organization of Vertebrate Pavlovian Conditioning. Cold Spring Harbor Perspectives in Biology, 8(1). doi:ARTN a021717 10.1101/cshperspect.a021717
Landeira-Fernandez, J., DeCola, J. P., Kim, J. J., & Fanselow, M. S. (2006). Immediate shock deficit in fear conditioning: effects of shock manipulations. Behav Neurosci, 120(4), 873-879. doi:10.1037/0735-7044.120.4.873
Lima, S. L., & Dill, L. M. (1990). Behavioral Decisions Made under the Risk of Predation - a Review and Prospectus. Canadian Journal of Zoology, 68(4), 619-640. doi:DOI 10.1139/z90-092
Maren, S. (1999). Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci, 19(19), 8696-8703.
Moita, M. A., Rosis, S., Zhou, Y., LeDoux, J. E., & Blair, H. T. (2003). Hippocampal place cells acquire location-specific responses to the conditioned stimulus during auditory fear conditioning. Neuron, 37(3), 485-497. doi:10.1016/s0896-6273(03)00033-3
Sigmundi, R. A., Bouton, M. E., & Bolles, R. C. (1980). Conditioned Freezing in the Rat as a Function of Shock-Intensity and Cs Modality. Bulletin of the Psychonomic Society, 15(4), 254-256.
Steimer, T. (2002). The biology of fear- and anxiety-related behaviors. Dialogues Clin Neurosci, 4(3), 231-249.
Stephens, D. W., & Krebs, J. R. (1986). Foraging Theory: Princeton University Press.
Tolman, E. C. (1948). Cognitive maps in rats and men. Psychol Rev, 55(4), 189-208. doi:10.1037/h0061626
Zambetti, P. R., Schuessler, B. P., & Kim, J. J. (2019). Sex Differences in Foraging Rats to Naturalistic Aerial Predator Stimuli. iScience, 16, 442-452. doi:10.1016/j.isci.2019.06.011
Zimmerman, J. M., Rabinak, C. A., McLachlan, I. G., & Maren, S. (2007). The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem, 14(9), 634-644. doi:10.1101/lm.607207
-

Evaluation Summary:
This paper is of potential interest to a broad audience of neuroscientists. By concluding that fear conditioning does not occur in a semi-naturalistic experimental setup, the study implies a major adjustment in our current understanding of Pavlovian fear conditioning and associative learning. However, additional controls and data analyses are required to validate the authors' conclusions.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This manuscript describes a series of behavioral experiments in which foraging rats are subjected to a novel fear conditioning paradigm. Different groups of animals receive a shock to the dorsal surface of the body paired with either tone, an artificial owl driven forward with pneumatic pressure, or a tone/owl combination. An additional control condition pairs tone with owl alone (ie no shock is delivered). In a subsequent test, only owl+shock and tone/owl+shock animals show increased latency to forage and a withdrawal response to tone (even though owl-shock rats do not experience tone during conditioning). The authors conclude that this tone response is due to sensitization and that fear conditioning does not occur in their experimental setup.
This approach is intriguing and the issues raised by the …
Reviewer #1 (Public Review):
This manuscript describes a series of behavioral experiments in which foraging rats are subjected to a novel fear conditioning paradigm. Different groups of animals receive a shock to the dorsal surface of the body paired with either tone, an artificial owl driven forward with pneumatic pressure, or a tone/owl combination. An additional control condition pairs tone with owl alone (ie no shock is delivered). In a subsequent test, only owl+shock and tone/owl+shock animals show increased latency to forage and a withdrawal response to tone (even though owl-shock rats do not experience tone during conditioning). The authors conclude that this tone response is due to sensitization and that fear conditioning does not occur in their experimental setup.
This approach is intriguing and the issues raised by the manuscript are extremely important for the field to consider. However, there are many ways to interpret the results as they stand. One issue of primary importance is whether it can indeed be claimed that conditioning did not readily occur in the tone+shock group. The lack of a particular behavioral conditioned reaction does not equate to an absence of conditioning. It is possible that unseen (i.e. physiological) measures of conditioning, many of which were once standard DVs in the fear conditioning literature, are present in the tone+shock group. This possibility pushes against the claim made in the title and elsewhere. These claims should be softened.
Because systemic, group-level retreat CRs are not noted in the tone+shock condition, it would indeed be important to establish if there are any experimental circumstances in which tone paired with a US applied to the dorsal surface of the body can produce consistent reactions (e.g. freezing) to tone alone. Though it may seem likely that tone + dorsal shock would indeed produce freezing in a different setting, this result should not be taken for granted - we've known since the 'noisy water' experiment (Garcia & Koelling, 1966) that not every CS pairs with every US and that association can indeed be selective. A positive control would be clarifying. If the authors could demonstrate that tone+dorsal shock produces freezing to tone in a commonly used fear conditioning setup (ie standard cubicle chamber) then the lack of a retreat CR in their naturalistic paradigm would gain added meaning.
The altered withdrawal trajectory seen in owl+shock and tone/owl+shock groups occurs in neither the tone+shock nor the tone+owl group, introducing the possibility that it results from the specific pairing of owl and shock. Put differently - this response may indeed by an associative CR. Do altered withdrawal angles persist if animals that receive owl+shock are exposed to owl again the next day? Do manipulations of the owl and shock that diminish fear conditioning (e.g. unpairing of owl and shock stimuli) eliminate deflected withdrawal angles when the subject is exposed to owl alone? If so, it would cut against the interpretation that fear conditioning does not occur in the setup described here, and would instead demonstrate that it is indeed central to predatory defense. This interpretation is compatible with the effect of hippocampal lesion on freezing evoked by a live predator. Destruction of the rat hippocampus diminishes cat-evoked freezing - this is thought to occur because the rapid association of the cat's various features with threatening action is not formed by the rat (Fanselow, 2000, 2018). Even though this interpretation of the results differs from the authors', it in no way diminishes the interest of this work. This paradigm may indeed be a novel means by which to study rapidly acquired associations with ethological relevance. Follow-up experiments of the type described above are necessary to disambiguate opposing views of the current dataset.
-

Reviewer #2 (Public Review):
This work is dealing with an interesting question whether a simple, one trial CS+US (Pavlovian) association occurs in a naturalistic environment. Pavlovian fear conditioning contains a repetition of a neutral sensory signal (tone, CS) which is paired with a mild US, usually foot-shock (<1 mA; thus, unpleasant rather than painful) and the CS+US association drives associative learning. In this paper, a single 2.5 mA electrical shock was paired with a novel 80 dB tone to monitor the occurrence of learning via measuring success rate and latency of foraging for food. Some animals experienced an owl-looming matched with the US, just before reaching the food. The authors placed hunger-motivated rats into a custom-built arena equipped with safe nest, gate, food zone as well as with a delivery of a self-controlled US …
Reviewer #2 (Public Review):
This work is dealing with an interesting question whether a simple, one trial CS+US (Pavlovian) association occurs in a naturalistic environment. Pavlovian fear conditioning contains a repetition of a neutral sensory signal (tone, CS) which is paired with a mild US, usually foot-shock (<1 mA; thus, unpleasant rather than painful) and the CS+US association drives associative learning. In this paper, a single 2.5 mA electrical shock was paired with a novel 80 dB tone to monitor the occurrence of learning via measuring success rate and latency of foraging for food. Some animals experienced an owl-looming matched with the US, just before reaching the food. The authors placed hunger-motivated rats into a custom-built arena equipped with safe nest, gate, food zone as well as with a delivery of a self-controlled US (electrical shock in the neck muscle and/or owl-looming). The US was activated by the rats by approaching to the food. Thus, a conflicting situation was provoked where procuring the food is paired with an aversive conditioned signal. Four groups of rats were included in the experiments based on their conditioning types: tone+ shock, tone+ shock+ owl, shock+owl and tone+owl. Due to these conditioning procedures, none of the rat procured the food but fled to the nest. In contrast, in the retrieval phases (next two days), the tone-shock and tone-owl groups successfully procured the pellets but not the tone-shock-owl group during the conditioned tone presentation. Rats in the latter group fled to the nest upon tone presentation at the food zone. As the shock-owl animals (conditioned without tone) also fled to the nest triggered by (unfamiliar) tone presentation, their and the tone+shock+owl group's fled responses were assigned to be non-associative sensitization-like process. Furthermore, during the pre-tone trials, all groups showed similar behavior as in the tone test. These findings led the authors to conclude that classical Pavlovian fear conditioning may not present in an ecologically relevant environment.
The raised question is relevant for broad audience of neuroscience and behavioral scientist. However, as the used fear conditioning paradigm is not a common one, it is difficult to interpret the finding. It is based on a single pairing of an unfamiliar, salient tone with a very strong (traumatizing?) electrical shock, delivered directly into the neck muscle and an innate signal (owl looming). In addition, as the tone presentation was followed by many events (gate opening, presence of food, shock and/or owl-looming) in front of the animals, it is hard to image what sort of tone association could be formed at all.
One could also argue that if a hungry animal does not try to collect food after an unpleasant, even a painful experience, then, it normally dies soon (thus, that is not a 'natural' behavior). The tone+shock and tone+owl groups showed similar behavioral features throughout the entire experiments and may reconcile the natural events: although these rats had had negative experience before, were still approaching to food zone due their hunger. Because of their motivation for food, the authors concluded that no association was formed. Based on this single measure, is it right to do so?
-

Reviewer #3 (Public Review):
In this study, the authors aimed to test whether rats could be fear conditioned by pairing a subdermal electric shock to a tone, an owl-like approaching stimulus, or a combination of these in a naturalistic-like environment. The authors designed a task in which rats foraging for food were exposed to a tone paired to a shock, an owl-like stimulus, a combination of the owl and the shock, or paired the owl to a shock in a single trial. The authors indexed behaviors related to food approach after conditioning. The authors found that animals exposed to the owl-shock or the tone/owl-shock pairing displayed a higher latency to approach the food reward compared to animals that were presented with the tone-shock or the tone-owl pairing. These results suggest that pairing the owl with the shock was sufficient to …
Reviewer #3 (Public Review):
In this study, the authors aimed to test whether rats could be fear conditioned by pairing a subdermal electric shock to a tone, an owl-like approaching stimulus, or a combination of these in a naturalistic-like environment. The authors designed a task in which rats foraging for food were exposed to a tone paired to a shock, an owl-like stimulus, a combination of the owl and the shock, or paired the owl to a shock in a single trial. The authors indexed behaviors related to food approach after conditioning. The authors found that animals exposed to the owl-shock or the tone/owl-shock pairing displayed a higher latency to approach the food reward compared to animals that were presented with the tone-shock or the tone-owl pairing. These results suggest that pairing the owl with the shock was sufficient to induce inhibitory avoidance, whereas a single pairing of the tone-shock or the tone-owl was not. The authors concluded that standard fear conditioning does not readily occur in a naturalistic-like environment and that the inhibitory avoidance induced by the owl-shock pairing could be the result of increased sensitization rather than a fear association.
Strengths:
The manuscript is well-written, the behavioral assay is innovative, and the results are interesting. The inclusion of both males and females, and the behavioral sex comparison was commendable. The findings are timely and would be highly relevant to the field.
Weaknesses:
However, in its current state, this study does not provide convincing evidence to support their main claim that Pavlovian fear conditioning does not readily occur in naturalistic environments. The innovative task presented in this study is more akin to an inhibitory avoidance task rather than fear conditioning and should be reframed in such way.
-