Minimal-invasive enhancement of auditory perception by terahertz wave modulation
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The work investigates cochlear physiology by applying terahertz wave modulation to the outer hair cells (OHCs). Improved cochlea sensitivity and a change in potassium membrane current is demonstrated. The work is of clear interest to auditory neuroscientists and has the potential for future clinical interest.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Article activity feed
-
-

Author Response:
Reviewer #1:
This study examines the use of terahertz wave modulation (THM), a technique for transmitting terahertz wave electromagnetic energy to the cochlea with the aim of improving the sensitivity of the cochlear outer hair cells. ABR obtained with and without THM suggests that sensitivity thresholds were improved by 10 dB when using THM. Whole-call patch clam recordings from outer hair cells suggest that THM significantly increases both K+ and MET currents of the cochlear outer hair cells. These results are convincing and potentially important for understanding normal cochlear physiology.
On the other hand, the numerous claims about translational applicability of this work seem overstated.
61-65 This is incorrect. For example, optogenetics or stem cell use are not currently seen as "treatment for hearing …
Author Response:
Reviewer #1:
This study examines the use of terahertz wave modulation (THM), a technique for transmitting terahertz wave electromagnetic energy to the cochlea with the aim of improving the sensitivity of the cochlear outer hair cells. ABR obtained with and without THM suggests that sensitivity thresholds were improved by 10 dB when using THM. Whole-call patch clam recordings from outer hair cells suggest that THM significantly increases both K+ and MET currents of the cochlear outer hair cells. These results are convincing and potentially important for understanding normal cochlear physiology.
On the other hand, the numerous claims about translational applicability of this work seem overstated.
61-65 This is incorrect. For example, optogenetics or stem cell use are not currently seen as "treatment for hearing impairment" and, in fact, the manuscript says as much later in the paragraph. Also, pharmacological treatment is rarely effective, and only in limited circumstances.
Many thanks to reviewers for pointing out this mistake, We have replaced the discussion by:
“At present, treatment for hearing impairment is primarily administered through pharmacological treatment, hearing aid equipment, and electronic cochlear implantation (Wilson et al., 1991; Kipping et al., 2020; Gang et al., 2008). Optogenetics (Huet et al., 2021), stem cell differentiation and transplantation (Oshima et al., 2010; Li et al., 2003; Chen et al., 2012) are also being explored to treat hearing loss. However, pharmacological treatment is rarely effective, and only in limited circumstances.”
283-294 The discussion of near-infrared vs THM is misguided. Near-infrared has been proposed as a possible alternative technology to stimulate spiral ganglion neurons, thus replacing cochlear implants. This is plausible, even though feasibility has not yet been demonstrated. In contrast, THM does not seem like a plausible alternative to cochlear implants. Patients who are candidates for cochlear implantation may not have enough (or any) outer hair cells, which are the target for THM.
Thank the reviewer for pointing out the difference in principle between Near-infrared auditory stimulation and THM. We have now modified the main text and compared the differences and similarities between THM and NIRS. Please see the revised Discussion.
295-299 "In comparison with wearing hearing aids, stem cell differentiation and transplantation (Oshima et al., 2010; Li et al., 2003; Chen et al., 2012), optogenetics (Huet et al., 2021) and electronic cochlear implantation (Wilson et al., 1991; Kipping et al., 2020; Gang et al., 2008), THM requires no traumatic surgery, cumbersome equipment, or genetic manipulation, and is thus more suitable for use in human subjects." In the described experiment, optic fibers had to be placed close to outer hair cells. That seems to require "cumbersome equipment" and obviously would require surgery for use in humans.
Many thanks to the reviewer for pointing out these inappropriate statement. We completely agree. We have now revised this statement in the revised manuscript.
The data show that sensitivity was improved by 8.75 dB. In practical terms this is a very small change. Sensitivity improvement of 10 dB (and much more than that) can be obtained non invasively and on a frequency dependent basis using traditional amplification.
Any neural stimulation technology would require not only spatial selectivity but also temporal responsiveness. It seems that THM could meet the former criteria but the latter is unknown. In other words, for any practical application it would be necessary to show that modulation of a THM signal can be perceived by listeners. However, this criticism is moot if the claims about clinical applicability of THM are removed.
We thank for the reviewer’s constructive comments. We completely agree with these comments and the claims about clinical applicability of THM are removed.
Reviewer #2:
This manuscript uses mid-infrared light to enhance the currents from natural stimuli (mechanical and voltage) of hair cells. The authors show increased voltage-gated K+ current and MET currents while being illuminated with mid-infrared light. Based on molecular dynamics simulations, the authors hypothesize that the augmented voltage-gated K+ currents are due to stimulation of C=O groups in the selectivity filter which allows K+ ions to pass through the pore more quickly to increase conductance; there was no hypothesis as to why MET currents were augmented. The authors also demonstrate improved ABR thresholds when the cochlea was illuminated with the mid-infrared light, demonstrating a potential therapeutic application. The enthusiasm for the novelty of this work is reduced because other work has shown that neurons can be excited by near-infrared (~2 microns) wavelength due to thermal stimulation and changes in cell capacitance, so this work mainly differs in their proposed mechanism and the longer wavelength of light (8.6 microns). Additionally, the Hudspeth group (Azimzadeh et al, 2018, PMC5805653) has shown thermal gating of MET channels using ultraviolet light and infrared light (1.47 microns). If the THM mechanism is indeed different from thermal stimulation, this would be a novel therapeutic mode, however, the data are not yet convincing that thermal stimulation is not the mechanism of action.
We thank the reviewer’s suggestions that are essential for improving our manuscript, in particular to pointing out the important literature about thermal gating of MET channels. We have now cited and discussed this review paper and other related papers.
Since the structure of the MET channels have not been resolved, we cannot study the mechanism at the atomic or chemical bond level by molecular dynamics.
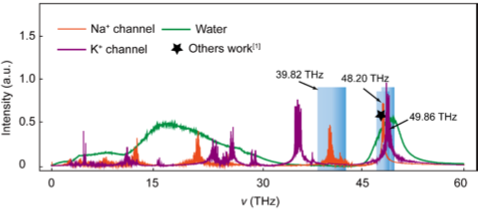
Infrared stimulation is emerging as an area of interest for neuromodulation and potential clinical application.While most studies on infrared stimulation have been conducted at near infrared wavelengths, whether mid-infrared wavelengths can impact neuronal function is unknown. A large number of studies have shown that the threshold of action potential generated by INS stimulation is correlated with the solution absorption coefficient to wavelength, that is, the higher the solution absorption coefficient is, the lower the threshold is. Therefore, the mechanism of action potential induced by INS is generally believed to be the rapid rise of solution temperature caused by INS, namely “ Photothermal effect ”[1]. However, as figure R1 shown, the absorption of water to the wavelength 8.6 μm we use is very weak.
How does near-infrared light affect the excitability of cells or nerves through “ photothermal effect ”, so as to promote the generation or propagation of action potential in neurons or inhibit the generation or propagation of action potential? In other words, what is the target of “ photothermal effect ” ? Currently, there are few studies on the mechanisms, and the possible biophysical mechanisms include the following three:
(1) After INS is absorbed by solution , the solution temperature increases rapidly, the membrane capacitance changes and the inward current is induced, which leads to the depolarization of membrane potential and the generation of action potential[2]; (2) INS activates temperature-sensitive TRP ion channels, which causes an action potential[3]; (3) INS enhanced inhibitory postsynaptic by acting on GABA receptor, thus producing inhibitory effect[4].
At present, the wavelength of INS is mainly near infrared light (1-3 microns), the parameters used are not consistant, and there are many factors affecting the excitation or inhibition of INS (such as the diameter of the fiber, the energy of infraredlight, pulse width, repetition frequency). On the one hand, photothermal effect is difficult to control, and some studies have found that overheating photothermal effect will block the generation and propagation of action potential, and even cause irreversible effects of INS on inhibition of action potential and tissue damage [5]. On the other hand, it is difficult to determine the target of photothermal action, which hinders the safe and effective promotion of INS as a neuroregulatory tool to the clinical or research field. Therefore, new regulatory strategies with more explicit mechanisms are needed in the field of photoneural regulation.
References:
Wells, J., Kao, C., Konrad, P., Milner, T., Kim, J., Mahadevan-Jansen, A., Jansen, E.D.: Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophysical Journal. 93, 2567-2580 (2007).
Shapiro, M.G., Homma, K., Villarreal, S., Richter, C.P., Bezanilla, F.: Infrared light excites cells by changing their electrical capacitance. Nature Communications. 3, (2012).
Albert, E.S., Bec, J.M., Desmadryl, G., Chekroud, K., Travo, C., Gaboyard, S., Bardin, F., Marc, I., Dumas, M., Lenaers, G., Hamel, C., Muller, A., Chabbert, C.: TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. Journal of Neurophysiology. 107, 3227–3234 (2012).
Feng, H.J., Kao, C., Gallagher, M.J., Jansen, E.D., Mahadevan-Jansen, A., Konrad, P.E., Macdonald, R.L.: Alteration of GABAergic neurotransmission by pulsed infrared laser stimulation. Journal of Neuroscience Methods. 192, 110–114 (2010).
Walsh, A.J., Tolstykh, G.P., Martens, S., Ibey, B.L., Beier, H.T.: Action potential block in neurons by infrared light. Neurophotonics. 3, 040501 (2016).
The authors hypothesize that the increase in K+ current through voltage gated channels is due to increasing the speed of movement of the K+ ions through the selectivity filter, which they modeled with molecular dynamics simulations. However, the simulations are not validated with experimental manipulations.
We thank the reviewer for pointing this out. As shown in Figure R1, we overlapped the vibration spectra of modeled channels and the attenuation of infrared light in water.
Figure. R1. Comparisons of the absorption intensity of water molecular (green curve), Na+ channel (orange curve), and K+ channel (black curve) from our MD simulation, and the values from other molecular dynamics calculations [1] (purple star), respectively.
As shown in the FIG. R1, the strong absorption of THz wave located at the frequency of 49.86 THz for K+ channel, but it falls in the strong absorption region of water molecules. Otherwise, THz wave modulation (THM) will be interfered with by the thermal effect caused by the large absorption of water molecules.
For Na+ channels, the strongest absorption peak is located at 48.20 THz, which is consistent with these calculation results reported in the references of <PNAS 118, e2015685118 (2021)>. Nevertheless, it falls in the absorption region of water molecules and can be preferentially large absorbed by water molecules. In theory, the frequency of 39.82 THz can avoid the absorption of water molecules and regulate the carboxyl (-COO-) groups of Na+ channels in a non-thermal way, thus promoting or inhibiting the Na+ current. Unfortunately, these results are difficult to be confirmed by experiment methods due to no strong enough of the intensity of light source corresponding to this frequency, so the laser cannot be effectively coupled to the optical fiber to focus on nerve cells, which affects the current test of ion channel under terahertz stimulations [2]. We believe that the regulation characteristics of terahertz waves with specific frequency on Na+ channels will be further studied when the light source and coupling technology of correlation frequency are well developed in the future.
References:
Xi Liu†, Zhi Qiao†, Yuming Chai†, Zhi Zhu†, Kaijie Wu, Wenliang Ji, Daguang Li, Yujie Xiao, Junlong Li, Lanqun Mao, Chao Chang, Quan Wen, Bo Song, Yousheng Shu, Non-thermal and reversible control of neuronal signaling and behavior by mid-infrared stimulation. Proc. Natl. Acad. Sci. U. S. A. 118 (10): e2015685118, (2021).
Seddon, Angela B. "Mid-infrared (MIR) photonics: MIR passive and active fiberoptics chemical and biomedical, sensing and imaging." Emerging Imaging and Sensing Technologies. International Society for Optics and Photonics, 9992, 999206, (2016).
It was unclear to this reviewer whether the temperature effect would be measurable with the technique used. It appears that the temperature measuring system is rather large as compared to the cell, therefore it would likely measure changes in bulk solution temperature and not necessarily a local or micro-scale change in temperature that the cell may be responding too. Additionally, Littlefield and Richter has suggested that temperature changes on the order of 0.1 degrees Celsius are sufficient to evoke action potentials (Littlefield & Richter, 2021, PMC8035937), which is well within the temperature changes observed by the authors. At the longer wavelengths used in this study, the absorption of water is generally even higher as well, suggesting even greater temperature changes with the same power. In vestibular hair cells a 10 deg Celsius increase in temperature led to a 50-60% increase in peak MET current (Songer & Eatock, 2013, PMC3857958).
We thank the reviewer for pointing out this issue. Indeed, the temperature measuring system is rather large as compared to the cell. we performed the temperature measurement protocal with an ADINSTRUMENT acquisition system (PowerLab 4/35) coupled to a T-type hypodermic thermocouple (MT 29/5, Physitemp),the diameter of the thermocouple is 100 μm. However, our new experiment on measuring tissue temperature in vitro showed that the maximum temperature elevation was less than 4 °C with the 75 mW stimulation, which was much lower than the temperature measured in the reference paper (10°C,Songer & Eatock, 2013, PMC3857958) and another paper (Littlefield & Richter, 2021, PMC8035937) mentioned by this reviewer also proposed in the introduction that light stimulation arouses neural responses due to photons rather than heat.. In addition, when the power is 10 mW, the temperature rise is not more than 1°C. two studies have found light illumination that is commonly used for optogenetics increases the temperature by ~2°C[1-2].This temperature elevation is associated with the inhibition of neuronal spiking in different brain areas and cannot explain the excitation effect observed in our experiment by the THM. We now mentioned this point in the main text. In addition, we also mention in the main text that the wavelength of 8.6 μm falls in the strong absorption region of water.
References:
- Owen, S. F., Liu, M. H. & Kreitzer, A. C. Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci. 22, 1061–1065 (2019)
- Ait Ouares, K., Beurrier, C., Canepari, M., Laverne, G. & Kuczewski, N. Opto nongenetics inhibition of neuronal firing. Eur. J. Neurosci. 49, 6–26 (2019).
In figure 1, when THM is on, there appears to be an increase in the inward current without any mechanical stimulation. There is no discussion of this, and this could be a baseline effect that is not aimed at simply enhancing existing conductances. The increase in K+ conductance seen in the voltage-gated K channel cannot account for this increased inward current, since K+ conductance is outward. THM itself could also activate a small amount of MET current, maybe via the thermal effect demonstrated by Azimzadeh et al. This increased conductance could also be from the Tmc1 leak conductance that the authors have published on previously.
We thank the reviewer for pointing out this issue, in particular for suggesting several possible reasons about the increase in the inward current. We have now discussed this effect and cited related papers. In addition, the increase in MET currents caused by THM was far greater than the baseline offset, indicating that THM has a non-thermal effect.
Line 232-233: With regard to the ABR data, data is not shown about whether an OABR can be elicited. The data show that once the THM is turned on and then a click stimulus is presented, there is no response; however, this experiment does not really test whether the THM can evoke an OABR since many repetitions are required to get the ABR waveform out of the noise. If THM is on and the stimulus is below threshold, then there is unlikely going to be an evoked response since the THM stimulus is not synchronized with the ABR recording. The authors need to show that THM onset stimulation that is synchronized with the ABR recording does not result in an ABR waveform.
We thank the reviewer for suggesting this very important experiment. Following this suggestion, we test whether the THM onset stimulation that is synchronized with the ABR recording can evoke an OABR. We now present the new data in Figure S5.
-

Evaluation Summary:
The work investigates cochlear physiology by applying terahertz wave modulation to the outer hair cells (OHCs). Improved cochlea sensitivity and a change in potassium membrane current is demonstrated. The work is of clear interest to auditory neuroscientists and has the potential for future clinical interest.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This study examines the use of terahertz wave modulation (THM), a technique for transmitting terahertz wave electromagnetic energy to the cochlea with the aim of improving the sensitivity of the cochlear outer hair cells. ABR obtained with and without THM suggests that sensitivity thresholds were improved by 10 dB when using THM. Whole-call patch clam recordings from outer hair cells suggest that THM significantly increases both K+ and MET currents of the cochlear outer hair cells. These results are convincing and potentially important for understanding normal cochlear physiology.
On the other hand, the numerous claims about translational applicability of this work seem overstated.
61-65 This is incorrect. For example, optogenetics or stem cell use are not currently seen as "treatment for hearing impairment" …
Reviewer #1 (Public Review):
This study examines the use of terahertz wave modulation (THM), a technique for transmitting terahertz wave electromagnetic energy to the cochlea with the aim of improving the sensitivity of the cochlear outer hair cells. ABR obtained with and without THM suggests that sensitivity thresholds were improved by 10 dB when using THM. Whole-call patch clam recordings from outer hair cells suggest that THM significantly increases both K+ and MET currents of the cochlear outer hair cells. These results are convincing and potentially important for understanding normal cochlear physiology.
On the other hand, the numerous claims about translational applicability of this work seem overstated.
61-65 This is incorrect. For example, optogenetics or stem cell use are not currently seen as "treatment for hearing impairment" and, in fact, the manuscript says as much later in the paragraph. Also, pharmacological treatment is rarely effective, and only in limited circumstances.
283-294 The discussion of near-infrared vs THM is misguided. Near-infrared has been proposed as a possible alternative technology to stimulate spiral ganglion neurons, thus replacing cochlear implants. This is plausible, even though feasibility has not yet been demonstrated. In contrast, THM does not seem like a plausible alternative to cochlear implants. Patients who are candidates for cochlear implantation may not have enough (or any) outer hair cells, which are the target for THM.
295-299 "In comparison with wearing hearing aids, stem cell differentiation and transplantation (Oshima et al., 2010; Li et al., 2003; Chen et al., 2012), optogenetics (Huet et al., 2021) and electronic cochlear implantation (Wilson et al., 1991; Kipping et al., 2020; Gang et al., 2008), THM requires no traumatic surgery, cumbersome equipment, or genetic manipulation, and is thus more suitable for use in human subjects." In the described experiment, optic fibers had to be placed close to outer hair cells. That seems to require "cumbersome equipment" and obviously would require surgery for use in humans.
The data show that sensitivity was improved by 8.75 dB. In practical terms this is a very small change. Sensitivity improvement of 10 dB (and much more than that) can be obtained non invasively and on a frequency dependent basis using traditional amplification.
Any neural stimulation technology would require not only spatial selectivity but also temporal responsiveness. It seems that THM could meet the former criteria but the latter is unknown. In other words, for any practical application it would be necessary to show that modulation of a THM signal can be perceived by listeners. However, this criticism is moot if the claims about clinical applicability of THM are removed.
-

Reviewer #2 (Public Review):
This manuscript uses mid-infrared light to enhance the currents from natural stimuli (mechanical and voltage) of hair cells. The authors show increased voltage-gated K+ current and MET currents while being illuminated with mid-infrared light. Based on molecular dynamics simulations, the authors hypothesize that the augmented voltage-gated K+ currents are due to stimulation of C=O groups in the selectivity filter which allows K+ ions to pass through the pore more quickly to increase conductance; there was no hypothesis as to why MET currents were augmented. The authors also demonstrate improved ABR thresholds when the cochlea was illuminated with the mid-infrared light, demonstrating a potential therapeutic application. The enthusiasm for the novelty of this work is reduced because other work has shown that …
Reviewer #2 (Public Review):
This manuscript uses mid-infrared light to enhance the currents from natural stimuli (mechanical and voltage) of hair cells. The authors show increased voltage-gated K+ current and MET currents while being illuminated with mid-infrared light. Based on molecular dynamics simulations, the authors hypothesize that the augmented voltage-gated K+ currents are due to stimulation of C=O groups in the selectivity filter which allows K+ ions to pass through the pore more quickly to increase conductance; there was no hypothesis as to why MET currents were augmented. The authors also demonstrate improved ABR thresholds when the cochlea was illuminated with the mid-infrared light, demonstrating a potential therapeutic application. The enthusiasm for the novelty of this work is reduced because other work has shown that neurons can be excited by near-infrared (~2 microns) wavelength due to thermal stimulation and changes in cell capacitance, so this work mainly differs in their proposed mechanism and the longer wavelength of light (8.6 microns). Additionally, the Hudspeth group (Azimzadeh et al, 2018, PMC5805653) has shown thermal gating of MET channels using ultraviolet light and infrared light (1.47 microns). If the THM mechanism is indeed different from thermal stimulation, this would be a novel therapeutic mode, however, the data are not yet convincing that thermal stimulation is not the mechanism of action.
The authors hypothesize that the increase in K+ current through voltage gated channels is due to increasing the speed of movement of the K+ ions through the selectivity filter, which they modeled with molecular dynamics simulations. However, the simulations are not validated with experimental manipulations.
It was unclear to this reviewer whether the temperature effect would be measurable with the technique used. It appears that the temperature measuring system is rather large as compared to the cell, therefore it would likely measure changes in bulk solution temperature and not necessarily a local or micro-scale change in temperature that the cell may be responding too. Additionally, Littlefield and Richter has suggested that temperature changes on the order of 0.1 degrees Celsius are sufficient to evoke action potentials (Littlefield & Richter, 2021, PMC8035937), which is well within the temperature changes observed by the authors. At the longer wavelengths used in this study, the absorption of water is generally even higher as well, suggesting even greater temperature changes with the same power. In vestibular hair cells a 10 deg Celsius increase in temperature led to a 50-60% increase in peak MET current (Songer & Eatock, 2013, PMC3857958).
In figure 1, when THM is on, there appears to be an increase in the inward current without any mechanical stimulation. There is no discussion of this, and this could be a baseline effect that is not aimed at simply enhancing existing conductances. The increase in K+ conductance seen in the voltage-gated K channel cannot account for this increased inward current, since K+ conductance is outward. THM itself could also activate a small amount of MET current, maybe via the thermal effect demonstrated by Azimzadeh et al. This increased conductance could also be from the Tmc1 leak conductance that the authors have published on previously.
Line 232-233: With regard to the ABR data, data is not shown about whether an OABR can be elicited. The data show that once the THM is turned on and then a click stimulus is presented, there is no response; however, this experiment does not really test whether the THM can evoke an OABR since many repetitions are required to get the ABR waveform out of the noise. If THM is on and the stimulus is below threshold, then there is unlikely going to be an evoked response since the THM stimulus is not synchronized with the ABR recording. The authors need to show that THM onset stimulation that is synchronized with the ABR recording does not result in an ABR waveform.
-