Roles of G-protein coupled receptors and mechanosensitive ion channels in pressure-induced chronotropy of lymphatic vessels
Curation statements for this article:-
Curated by eLife
eLife Assessment
Davis and colleagues describe findings that are fundamental to the understanding of pressure mechanosensation in lymphatic vessels and are of significant importance to other areas of mechanosensory physiology. Based on many different knockout mouse models and rigorous state-of-the-art pressure myography recordings, they present compelling evidence that mechano-activation of GNAQ/GNA11-coupled GPCRs generates IP3, which induces Ca2+ release from internal stores through IP3R1 and drives depolarization through the activation of ANO1 Cl- channels to induce lymphatic vessel contractility. Nevertheless, some aspects of the manuscript are incomplete. The specific identity of the GPCR(s) involved remains to be uncovered, as evidence of frequency-pressure impairment is only demonstrated with abolition of GNAQ/GNA11action, not the receptors per se.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Active lymph pumping relies on the spontaneous contractions of collecting lymphatic vessels, with frequencies that are exquisitely sensitive to changes in intraluminal pressure. This homeostatic and mechanosensitive mechanism, termed pressure-induced lymphatic chronotropy, enables lymph transport to be matched to the filling state of the lymphatic capillaries. We investigated the mechanistic basis of pressure-induced chronotropy using ex vivo contraction assays of mouse popliteal collecting vessels, in which contraction frequency increased >10-fold with a 5 cmH2O pressure change. The contractile, electrophysiological and transcriptional similarities between lymphatic muscle and arterial smooth muscle led us to hypothesize that pressure-dependent chronotropy shares a parallel signaling process to pressure-induce arterial depolarization/constriction. Thus, we investigated two major mechanisms: 1) pressure-induced activation of mechanosensitive cation channels, including TRPC6, TRPM4, PKD1/2, TRPV2 and ENaC, and 2) mechano-activation of GNAQ/GNA11-coupled GPCRs that would generate second messengers to activate those channels. We combined contraction assays with scRNAseq analysis of the respective targets and made maximum use of transgenic mice to avoid non-specific effects of pharmacological inhibitors, particularly those used to block TRP channels. Our findings rule out significant roles for TRP and other mechanosensitive channels implicated in myogenic constriction, as well as channels implicated in ionic pacemaking of other tissues, and instead support a scheme whereby mechano-activation of GNAQ/GNA11-coupled GPCRs generates IP3, which induces SR Ca2+ release through IP3R1 and drives depolarization through the activation of ANO1 Cl− channels.
Article activity feed
-

Author response:
We thank the reviewers and editors for their insightful comments on our manuscript. We intend to submit a revised manuscript that addresses all concerns raised by the reviewers. A major limitation identified by the reviewers was our inability to identify one or more specific mechanosensitive GPCRs in lymphatic muscle cells (LMCs). To address this concern, we plan to include several additional figures in the revised manuscript. One figure will list the 136 GPCRs identified in LMCs by our scRNAseq analysis, based on the list of validated GPCRs in https://esbl.nhlbi.nih.gov/Databases/GPCRs/index.html and olfactory GPCRs listed in https://esbl.nhlbi.nih.gov/Databases/GPCRs/MouseHumanRatORs.html. We plan to arrange the data in a hierarchical manner according to their expression level and denote their heterotrimeric …
Author response:
We thank the reviewers and editors for their insightful comments on our manuscript. We intend to submit a revised manuscript that addresses all concerns raised by the reviewers. A major limitation identified by the reviewers was our inability to identify one or more specific mechanosensitive GPCRs in lymphatic muscle cells (LMCs). To address this concern, we plan to include several additional figures in the revised manuscript. One figure will list the 136 GPCRs identified in LMCs by our scRNAseq analysis, based on the list of validated GPCRs in https://esbl.nhlbi.nih.gov/Databases/GPCRs/index.html and olfactory GPCRs listed in https://esbl.nhlbi.nih.gov/Databases/GPCRs/MouseHumanRatORs.html. We plan to arrange the data in a hierarchical manner according to their expression level and denote their heterotrimeric GTP-binding protein alpha subunit(s), if known. To reinforce our finding that pressure-induced chronotropy in LMCs is mediated through Gq/11, we will present additional data testing the effects of acute Gq/11 inhibition with YM-254890 (a selective Gq/11 inhibitor) on the frequency-pressure relationship of popliteal vessels, as suggested by one reviewer. We will address concerns regarding the potential regional differences in lymphatic contractile regulation arising from our use of popliteal lymphatic vessels for contraction assays and expression analysis of LMCs obtained from Inguinal-Axillary lymphatic vessels (IALVs). To account for possible differences between the two, we will test pressure responses of IALVs from double Gq/11 knockout mice and test responses of wild-type IALVs to acute administration of YM-25489.
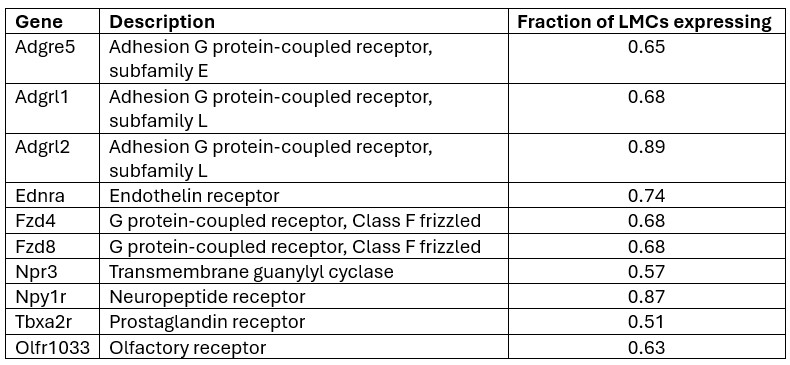
Our preliminary analysis of the 136 GPCRs in LMCs revealed a shorter list of 10 GPCRs that are expressed in at least 50% of LMCs (based on the IALV scRNAseq dataset). Since existing evidence from our studies, and those of other investigators, suggests that any LMC is capable of initiating pacemaking, we consider it reasonable to impose this requirement.
Author response table 1.
We plan to use pharmacologic inhibitors to test as many of these candidates as possible. Unfortunately, inhibitors are not available for many of the GPCRs listed above, but we will test Npr3, Npy1R, and Ednra; a negative result for Tbxa2r has already been documented in a previous study (Schulz et al. ATVB 2025). Even if this strategy does not lead to identification of one or more specific GPCRs involved in LMC pressure transduction, it will narrow the list of possible candidates that need to be tested in future experiments.
-

-

-

eLife Assessment
Davis and colleagues describe findings that are fundamental to the understanding of pressure mechanosensation in lymphatic vessels and are of significant importance to other areas of mechanosensory physiology. Based on many different knockout mouse models and rigorous state-of-the-art pressure myography recordings, they present compelling evidence that mechano-activation of GNAQ/GNA11-coupled GPCRs generates IP3, which induces Ca2+ release from internal stores through IP3R1 and drives depolarization through the activation of ANO1 Cl- channels to induce lymphatic vessel contractility. Nevertheless, some aspects of the manuscript are incomplete. The specific identity of the GPCR(s) involved remains to be uncovered, as evidence of frequency-pressure impairment is only demonstrated with abolition of GNAQ/GNA11action, not …
eLife Assessment
Davis and colleagues describe findings that are fundamental to the understanding of pressure mechanosensation in lymphatic vessels and are of significant importance to other areas of mechanosensory physiology. Based on many different knockout mouse models and rigorous state-of-the-art pressure myography recordings, they present compelling evidence that mechano-activation of GNAQ/GNA11-coupled GPCRs generates IP3, which induces Ca2+ release from internal stores through IP3R1 and drives depolarization through the activation of ANO1 Cl- channels to induce lymphatic vessel contractility. Nevertheless, some aspects of the manuscript are incomplete. The specific identity of the GPCR(s) involved remains to be uncovered, as evidence of frequency-pressure impairment is only demonstrated with abolition of GNAQ/GNA11action, not the receptors per se.
-

Reviewer #1 (Public review):
Summary:
Davis and co-authors used many mouse models to investigate mechanisms that regulate the contractility of mouse popliteal collecting vessels, primarily chronotropy. Many of the mechanisms studied were previously shown to regulate pressure-induced constriction in small arteries. The authors use prior literature from the vasculature as a framework to test similar concepts in lymphatic vessels. The mouse models used provide evidence for and against the involvement of multiple proteins in regulating chronotropy and other contractile properties in lymphatic vessels. They propose that mechano-activation of GNAQ/GNA11-coupled GPCRs generates IP3, which induces Ca2+ release through IP3R1 and drives depolarization through the activation of ANO1 Cl- channels. Major concerns include the author's major …
Reviewer #1 (Public review):
Summary:
Davis and co-authors used many mouse models to investigate mechanisms that regulate the contractility of mouse popliteal collecting vessels, primarily chronotropy. Many of the mechanisms studied were previously shown to regulate pressure-induced constriction in small arteries. The authors use prior literature from the vasculature as a framework to test similar concepts in lymphatic vessels. The mouse models used provide evidence for and against the involvement of multiple proteins in regulating chronotropy and other contractile properties in lymphatic vessels. They propose that mechano-activation of GNAQ/GNA11-coupled GPCRs generates IP3, which induces Ca2+ release through IP3R1 and drives depolarization through the activation of ANO1 Cl- channels. Major concerns include the author's major conclusion that GNAQ/GNA11-coupled GPCRs contribute to chronotropy. This conclusion is not supported by the data presented.
Strengths:
One major strength of the study lies in the vast number of mouse knockout models that were used to test the importance of ion channels and G protein signaling pathways in the regulation of lymphatic vessel contractility. In this regard, the study is a valiant effort. The authors achieved several objectives to find that ANO1 and IP3R1 regulate chronotropy, and many other potential proteins do not regulate chronotropy. This study will have a major impact on the field if additional support for G proteins is provided.
Weaknesses:
Major conclusions concerning the involvement of G proteins are drawn from the global Gna11 knockout mouse models. This conclusion is weak. Global Gna11 knockout mice are highly likely to have a multifactorial phenotype that could create significant differences in the data. Control experiments need to be performed on vessels from the global knockout mice if these major conclusions are to be made. Similarly, pharmacological tools or alternative approaches to manipulate G proteins should be used to support the data from these mouse models to draw these major conclusions.
The Gnaq smKO mice are the most specific G protein model studied here. However, there is no phenotype. Do not discuss trends in the data. If the data are not significant, conclude so. If more experiments are required to reach significance, provide more data in the manuscript.
The conclusions repeatedly refer to a signaling pathway wherein the upstream component is GPCRs, which activate G proteins. While this may be the case, no GPCRs were identified here, and the involvement of G proteins is questionable, as the authors outline in lines 693-695 and noted above. The conclusions should be tempered, including in the abstract, unless additional experiments are performed to support the involvement of G proteins. Perhaps then the authors may be able to infer that GPCRs are involved.
Line 318. The point regarding the choice to use popliteal vessels versus IALVs will be unclear to the uninitiated, particularly as the authors previously used IALVs. Including additional justification in the text and/or data from IALVs in Figure 1, which compares IALVs to popliteal vessels, would better explain the logic.
The conclusions drawn for TRPC6 and TRPC3 are less convincing. Germline global knockout mice, which are known to undergo compensation, were used, and high data variability is apparent. Using TRPC3 and TRPC6 blockers in the mouse models studied in Figure 4 would strengthen the arguments made regarding these proteins.
Did you perform power analysis to ensure that experimental numbers were sufficient to conclude that no statistical difference exists between datasets? If not, this needs to be done. For example, data shown in Figure 5C for tone and 6C for frequency and tone appear to be significantly different, but are concluded not to be so.
At the end of each result section, a concluding statement is made regarding the effects on pressure-induced chronotrophy. In many cases, there are additional effects of manipulating protein expression on other contractile properties. One example is for TRPC3 and TRPC6 (lines 414-416), but others are TRPV4, TRPV3, ENaC, Kir, Cav3.1/3.2, etc. Some interpretation is in the Discussion, but the concluding statements at the end of each result section should be expanded to summarize what the authors think the other significant differences in the data represent.
Kv7.4 channels. You state you have data (not shown) with linopiridine and XE991. Why not show those results here to support the experiments with the Kcnq4 smKO mice? Otherwise, I suggest you remove the statement from the unpublished data.
Figure 13A. Kcnj2 is modestly expressed in LECs, but very little is present in LMCs. This likely underlies the effect of barium. If you remove the endothelium, does the effect of barium disappear? While this is not the major focus of the study, the effects of barium are dramatic, and it should be made clear whether this is due to inhibition of Kir channels in smooth muscle or endothelial cells.
Figure 18C tone. Several values for losartan look different but are not labelled as such. Please clarify and discuss if different.
The manuscript should include raw data traces in figures that show the major pathways that you conclude regulate chronotropy.
-

Reviewer #2 (Public review):
Summary:
In this study, Davis et al. embarked on the quest for the molecular elements responsible for the regulation of lymphatic phasic contractile activity in response to variation of transmural pressure, a mechanism (termed pressure-induced lymphatic chronotropy by the authors) critical for drainage of interstitial fluid from the tissue and transport of lymph back to the blood circulation. Their aim was to investigate the mechanism(s) involved in the pressure-induced regulation of lymphatic pumping, and test whether activation of cation channels, shown in other systems to play mechanosensitive roles are directly at play, and/or whether mechano-activation of GNAQ/GNA11-coupled GPCRs is necessary to generate second messengers to activate those channels, as it has been suggested for the regulation of …
Reviewer #2 (Public review):
Summary:
In this study, Davis et al. embarked on the quest for the molecular elements responsible for the regulation of lymphatic phasic contractile activity in response to variation of transmural pressure, a mechanism (termed pressure-induced lymphatic chronotropy by the authors) critical for drainage of interstitial fluid from the tissue and transport of lymph back to the blood circulation. Their aim was to investigate the mechanism(s) involved in the pressure-induced regulation of lymphatic pumping, and test whether activation of cation channels, shown in other systems to play mechanosensitive roles are directly at play, and/or whether mechano-activation of GNAQ/GNA11-coupled GPCRs is necessary to generate second messengers to activate those channels, as it has been suggested for the regulation of myogenic tone in arteries. To achieve their goal, the authors used their well-described, highly reliable protocols of mouse lymphatic vessel isolation, pressure myography, and data acquisition to obtain frequency-pressure relationships and other contractile function parameters from transgenic mice where specific channels or molecular elements of interest have been ablated. They combined these data with scRNAseq analysis of these gene targets to determine their respective role and levels of expression in lymphatic muscle cells. Their conclusion is that none of the exhaustive list of tested ion channels was critical, except ANO1 Cl channels, part of the contractile pacemaker mechanism, but that transmural pressure activates GNAQ/GNA11-coupled GPCRs, which generate IP3 to induce SR Ca2+ release through IP3R1 and activate ANO1-mediated depolarization.
Strengths:
The manuscript's strengths reside primarily in very robust, clean, and unequivocal pressure myography data and analysis. The research team is mastering these techniques they developed more than a decade ago and have implemented in mouse lymphatics to study their contractile properties, with consistent and convincing outcomes. They also provide data from an impressive list of transgenic mice in order to determine the role of the targeted gene in pressure-induced lymphatic chronotropy, relying on pharmacological small molecule inhibitors only when necessary. Finally, the use of scRNAseq analysis they gathered from previously published datasets brings novelty with respect to the expression of the genes of interest in all populations of cells comprising the lymphatic vessels, but more critically, to validate or contrast the potential impact of genetic alteration of the given gene on the ability of lymphatic muscles to respond to a change in pressure.
Weaknesses:
The main weakness may reside in the fact that while the authors provide a convincing demonstration that GNAQ/GNA11 are involved in the regulation of the F-P relationship, they give little evidence of the involvement of "upstream" receptors. Indeed, inhibition of AT1R, shown to be involved in myogenic regulation of arteries (a phenomenon the authors rightfully compare to pressure-induced lymphatic chronotropy), didn't lead toa similar effect (decrease in F-P) in lymphatic vessels. Arguably, other GPCRs might be involved in lymphatic vessels, but as such information is not provided in the manuscript, the author's conclusions should be dampened. More in-depth discussion would be required. In fact, it can be argued that the discussion is very restricted with respect to the amount of data and information the manuscript provides.
Overall, the authors convincingly achieved their aim by performing an impressive number of technically challenging experiments, leading to solid datasets. While these support their main conclusions, a more elaborate discussion might be required to refine them.
This study is likely to have an important impact on the field as it provides some answers to the lingering question of how lymphatic vessels regulate their contractile activity to variation in transmural pressure and certainly proposes an experimental means to further explore and address that question.
-

Reviewer #3 (Public review):
In this manuscript, Davis and colleagues aimed to identify the molecular sensors and signaling cascade that enable collecting lymphatic vessels to increase their spontaneous contraction frequency in response to intraluminal pressure (pressure-induced chronotropy). They tested whether the process is similar to blood vessel myogenic constriction by relying on cation channels (TRPC6, TRPM4, PKD2, PIEZO1, etc.) or instead require the activation of G-protein-coupled receptors (presumably mechanosensitive GNAQ/GNA11-coupled receptors), using ex vivo pressure myography of mouse popliteal lymphatics, smooth muscle-specific conditional knockouts, quantitative PCR validation, and single-cell RNA sequencing for target prioritization. The authors convincingly demonstrate that pressure-induced chronotropy does not …
Reviewer #3 (Public review):
In this manuscript, Davis and colleagues aimed to identify the molecular sensors and signaling cascade that enable collecting lymphatic vessels to increase their spontaneous contraction frequency in response to intraluminal pressure (pressure-induced chronotropy). They tested whether the process is similar to blood vessel myogenic constriction by relying on cation channels (TRPC6, TRPM4, PKD2, PIEZO1, etc.) or instead require the activation of G-protein-coupled receptors (presumably mechanosensitive GNAQ/GNA11-coupled receptors), using ex vivo pressure myography of mouse popliteal lymphatics, smooth muscle-specific conditional knockouts, quantitative PCR validation, and single-cell RNA sequencing for target prioritization. The authors convincingly demonstrate that pressure-induced chronotropy does not require the cation channels implicated in arterial myogenic tone but is blunted by deletion of GNAQ/GNA11 or IP3 receptor 1, supporting a model of GPCR > IP3 > Ca2+ release > Cl⁻ channel activation > depolarization. The core conclusion is robust. The work redefines lymphatic pacemaking as G-protein-coupled receptor-dependent mechanotransduction, distinct from arterial mechanisms, and provides a genetically validated toolkit that is useful for studying lymphatic function and dysfunction.
Strengths:
(1) The data are of high quality and highly sensitive functional readouts
(2) The systematic genetic targeting is a major strength that overcomes pharmacological artifacts
(3) Careful quantitative analyses of frequency-pressure slopes
Weaknesses:
(1) The use of inguinal-axillary vessels for single-cell RNA sequencing rather than the popliteal segment studied functionally.
(2) No direct testing of the specific G-protein-coupled receptor involved.
-