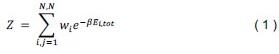Protein-Induced Membrane Strain Drives Supercomplex Formation
Curation statements for this article:-
Curated by eLife
eLife Assessment
In this important study, the authors conducted extensive atomistic and coarse-grained simulations as well as a lattice Monte Carlo analysis to probe the driving force and functional impact of supercomplex formation in the inner mitochondrial membrane. The study highlighted the major contribution from membrane mechanics to the supercomplex formation and revealed interesting differences in structural and dynamical features of the protein components upon complex formation. Upon revision, the analysis is considered solid, although the magnitude of estimated membrane deformation energies seem somewhat large. Overall, the study is thorough, creative and the impact on the field of bioenergetics is expected to be significant.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Mitochondrial membranes harbor the electron transport chain (ETC) that powers oxidative phosphorylation (OXPHOS) and drives the synthesis of ATP. Yet, under physiological conditions, the OXPHOS proteins operate as higher-order supercomplex (SC) assemblies, although their functional role remains poorly understood and much debated. By combining large-scale atomistic and coarse-grained molecular simulations with analysis of cryo-electron microscopic data and statistical as well as kinetic models, we show here that the formation of the mammalian I/III2 supercomplex reduces the molecular strain of inner mitochondrial membranes by altering the local membrane thickness and leading to an accumulation of both cardiolipin and quinone around specific regions of the SC. We find that the SC assembly also affects the global motion of the individual ETC proteins with possible functional consequences. On a general level, our findings suggest that molecular crowding and strain effects provide a thermodynamic driving force for the SC formation, with a possible flux enhancement in crowded biological membranes under constrained respiratory conditions.
Article activity feed
-

-

-

eLife Assessment
In this important study, the authors conducted extensive atomistic and coarse-grained simulations as well as a lattice Monte Carlo analysis to probe the driving force and functional impact of supercomplex formation in the inner mitochondrial membrane. The study highlighted the major contribution from membrane mechanics to the supercomplex formation and revealed interesting differences in structural and dynamical features of the protein components upon complex formation. Upon revision, the analysis is considered solid, although the magnitude of estimated membrane deformation energies seem somewhat large. Overall, the study is thorough, creative and the impact on the field of bioenergetics is expected to be significant.
-

Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written.
* Analysis of the bilayer curvature is challenging on the fine lengthscales they have used and produces unexpectedly large energies (Table 1). Additionally, the authors use the mean curvature (Eq. S5) as input to the (uncited, but it seems clear that this is Helfrich) Helfrich Hamiltonian (Eq. S7). If an errant factor of one half has been included with curvature, this would quarter the curvature energy compared to the real energy, due to the squared curvature. The bending modulus used (ca. 5 kcal/mol) is small on the scale of typically observed biological bending …
Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written.
* Analysis of the bilayer curvature is challenging on the fine lengthscales they have used and produces unexpectedly large energies (Table 1). Additionally, the authors use the mean curvature (Eq. S5) as input to the (uncited, but it seems clear that this is Helfrich) Helfrich Hamiltonian (Eq. S7). If an errant factor of one half has been included with curvature, this would quarter the curvature energy compared to the real energy, due to the squared curvature. The bending modulus used (ca. 5 kcal/mol) is small on the scale of typically observed biological bending moduli. This suggests the curvature energies are indeed much higher even than the high values reported. Some of this may be due to the spontaneous curvature of the lipids and perhaps the effect of the protein modifying the nearby lipids properties.
* It is unclear how CDL is supporting SC formation if its effect stabilizing the membrane deformation is strong or if it is acting as an electrostatic glue. While this is a weakness for a definite quantification of the effect of CDL on SC formation, the study presents an interesting observation of CDL redistribution and could be an interesting topic for future work.
In summary, the qualitative data presented are interesting (especially the combination of molecular modeling with simpler Monte Carlo modeling aiding broader interpretation of the results). The energies of the membrane deformations are quite large. This might reflect the roles of specific lipids stabilizing those deformations, or the inherent difficulty in characterizing nanometer-scale curvature.
-

Reviewer #3 (Public review):
Summary:
In this contribution, the authors report atomistic, coarse-grained and lattice simulations to analyze the mechanism of supercomplex (SC) formation in mitochondria. The results highlight the importance of membrane deformation as one of the major driving forces for the SC formation, which is not entirely surprising given prior work on membrane protein assembly, but certainly of major mechanistic significance for the specific systems of interest.
Strengths:
The combination of complementary approaches, including an interesting (re)analysis of cryo-EM data, is particularly powerful, and might be applicable to the analysis of related systems. The calculations also revealed that SC formation has interesting impacts on the structural and dynamical (motional correlation) properties of the individual protein …
Reviewer #3 (Public review):
Summary:
In this contribution, the authors report atomistic, coarse-grained and lattice simulations to analyze the mechanism of supercomplex (SC) formation in mitochondria. The results highlight the importance of membrane deformation as one of the major driving forces for the SC formation, which is not entirely surprising given prior work on membrane protein assembly, but certainly of major mechanistic significance for the specific systems of interest.
Strengths:
The combination of complementary approaches, including an interesting (re)analysis of cryo-EM data, is particularly powerful, and might be applicable to the analysis of related systems. The calculations also revealed that SC formation has interesting impacts on the structural and dynamical (motional correlation) properties of the individual protein components, suggesting further functional relevance of SC formation. In the revision, the authors further clarified and quantified their analysis of membrane responses, leading to further insights into membrane contributions. They have also toned down the decomposition of membrane contributions into enthalpic and entropic contributions, which is difficult to do. Overall, the study is rather thorough, highly creative and the impact on the field is expected to be significant.
Weaknesses:
Upon revision, I believe the weakness identified in previous work has been largely alleviated.
-

Author response:
The following is the authors’ response to the previous reviews
Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written.
We thank the Reviewer for finding our work interesting.
Analysis of the bilayer curvature is challenging on the fine lengthscales they have used and produces unexpectedly large energies (Table 1). Additionally, the authors use the mean curvature (Eq. S5) as input to the (uncited, but it seems clear that this is Helfrich) Helfrich Hamiltonian (Eq. S7). If an errant factor of one half has been included with curvature, this would quarter the curvature energy compared to the real …
Author response:
The following is the authors’ response to the previous reviews
Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written.
We thank the Reviewer for finding our work interesting.
Analysis of the bilayer curvature is challenging on the fine lengthscales they have used and produces unexpectedly large energies (Table 1). Additionally, the authors use the mean curvature (Eq. S5) as input to the (uncited, but it seems clear that this is Helfrich) Helfrich Hamiltonian (Eq. S7). If an errant factor of one half has been included with curvature, this would quarter the curvature energy compared to the real energy, due to the squared curvature.
We thank the Reviewer for raising this important issue. We have now clarified in the SI and main manuscript that we employ the Helfrich model. In our initial implementation, we indeed used the mean curvature H, thereby missing a factor of 2. As the Reviewer correctly noted, this resulted in curvature deformation energies that were underestimated by a factor of ~4. We have now corrected for this effect in the revised analysis, and the updated Table 1. Importantly, however, this correction does not alter the general conclusions of our work that supercomplex formation relieves membrane strain and stabilizes the system. We have added an additional paragraph where we discuss the magnitude of the observed bending effects, and compared the previous estimates in literature:
SI:
“The local mean curvature of the membrane midplane was computed using the Helfrich model (4,5) …”
(4) W. Helfrich, Elastic properties of lipid bilayers theory and possible experiments. Zeitschrift für Naturforschung 28c, 693-703 (1973).
(5) F. Campelo et al., Helfrich model of membrane bending: From Gibbs theory of liquid interfaces to membranes as thick anisotropic elastic layers. Advances in Colloid and Interface Science 208, 25-33 (2014).
Main Text:
“which measures the energetic cost of deforming the membrane from a flat geometry (ΔGcurv) based on the Helfrich model (45, 46). …
Our analysis suggests that both contributions are substantially reduced upon formation of the SC, with the curvature penalty decreasing by 79.2 ± 5.2 kcal mol-1 (for a membrane area of ca. 1000 nm2) and the thickness penalty by 2.8 ± 2.0 kcal mol-1 (Table 1).”
“We note that the magnitude of the estimated bending energies (~10² kcal mol-1) (Table 1), while seemingly high at first glance, falls within the range expected for large-scale membrane deformation processes induced by large multi-domain proteins. For example, the Piezo mechanosensitive channel performs roughly 150kBT (≈ 90 kcal mol⁻¹) of work to bend the bilayer into its dome-like shape (65). Comparable energies have also been estimated for the nucleation of small membrane pores (66), while vesicle formation typically requires bending energies on the order of 300 kcal mol-1, largely independent of vesicle size (67). When normalized by the affected membrane area (~1000 nm2), these values correspond to an energy density of approximately 0.1 kcal mol-1 nm-2, which places our estimates within a biophysically reasonable regime. Notably, cryo-EM structures of several supercomplexes shows that such assemblies can impose significant curvature on the surrounding bilayer (36, 50, 68), supporting the notion that respiratory chain organization is closely coupled to local membrane deformation. Nevertheless, we expect that the absolute deformation energies may be overestimated, as the continuum Helfrich model neglects molecular-level effects such as lipid tilt and local rearrangements, which can partially relax curvature stresses and reduce the effective bending penalty near protein–membrane interfaces (69, 70).”
The bending modulus used (ca. 5 kcal/mol) is small on the scale of typically observed biological bending moduli. This suggests the curvature energies are indeed much higher even than the high values reported. Some of this may be due to the spontaneous curvature of the lipids and perhaps the effect of the protein modifying the nearby lipids properties.
The SI initially included an incorrect value for the bending modulus (20 kJ mol-1 instead of 20kBT), which has now been corrected. The revised value is consistent with experimentally reported bending moduli from X-ray scattering measurements, although there remains substantial uncertainty in the precise values across different experimental and computational studies.
“The bending deformation energy was computed from the mean curvature field H(x,y), assuming a constant bilayer bending modulus κ (taken as 20kbT = 11.85 kcal mol-1 (6)):”
(6) S. Brown et al., Comparative analysis of bending moduli in one-component membranes via coarsegrained molecular dynamics simulations. Biophysical Journal 124, 1–13 (2025).
It is unclear how CDL is supporting SC formation if its effect stabilizing the membrane deformation is strong or if it is acting as an electrostatic glue. While this is a weakenss for a definite quantification of the effect of CDL on SC formation, the study presents an interesting observation of CDL redistribution and could be an interesting topic for future work.
We agree with the Reviewer that future studies would be important to investigate the relationship between CDL-induced stabilization of membrane and its electrostatic effects.
In summary, the qualitative data presented are interesting (especially the combination of molecular modeling with simpler Monte Carlo modeling aiding broader interpretation of the results). The energies of the membrane deformations are quite large. This might reflect the roles of specific lipids stabilizing those deformations, or the inherent difficulty in characterizing nanometer-scale curvature.
We thank the Reviewer for appreciating our work and for the help in further improving our findings.
Reviewer #3 (Public review):
Summary:
In this contribution, the authors report atomistic, coarse-grained and lattice simulations to analyze the mechanism of supercomplex (SC) formation in mitochondria. The results highlight the importance of membrane deformation as one of the major driving forces for the SC formation, which is not entirely surprising given prior work on membrane protein assembly, but certainly of major mechanistic significance for the specific systems of interest.
We thank Reviewer 3 for appreciating the importance of our study.
Strengths:
The combination of complementary approaches, including an interesting (re)analysis of cryo-EM data, is particularly powerful, and might be applicable to the analysis of related systems. The calculations also revealed that SC formation has interesting impacts on the structural and dynamical (motional correlation) properties of the individual protein components, suggesting further functional relevance of SC formation. In the revision, the authors further clarified and quantified their analysis of membrane responses, leading to further insights into membrane contributions. They have also toned down the decomposition of membrane contributions into enthalpic and entropic contributions, which is difficult to do. Overall, the study is rather thorough, highly creative and the impact on the field is expected to be significant.
Weaknesses:
Upon revision, I believe the weakness identified in previous work has been largely alleviated.
We thank the Reviewer for their previous remarks, which allowed us to significantly improve our manuscript.
-

-

eLife Assessment
In this important study, the authors conducted extensive atomistic and coarse-grained simulations as well as a lattice Monte Carlo analysis to probe the driving force and functional impact of supercomplex formation in the inner mitochondrial membrane. The study highlighted the major contribution from membrane mechanics to the supercomplex formation and revealed interesting differences in structural and dynamical features of the protein components upon complex formation. Upon revision, the analysis is considered solid, although the magnitude of estimated membrane deformation energies seem somewhat surprisingly large. Overall, the study is thorough, creative and the impact on the field of bioenergetics is expected to be significant.
-

Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written.
* Analysis of the bilayer curvature is challenging on the fine lengthscales they have used and produces unexpectedly large energies (Table 1). Additionally, the authors use the mean curvature (Eq. S5) as input to the (uncited, but it seems clear that this is Helfrich) Helfrich Hamiltonian (Eq. S7). If an errant factor of one half has been included with curvature, this would quarter the curvature energy compared to the real energy, due to the squared curvature. The bending modulus used (ca. 5 kcal/mol) is small on the scale of typically observed biological bending …
Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written.
* Analysis of the bilayer curvature is challenging on the fine lengthscales they have used and produces unexpectedly large energies (Table 1). Additionally, the authors use the mean curvature (Eq. S5) as input to the (uncited, but it seems clear that this is Helfrich) Helfrich Hamiltonian (Eq. S7). If an errant factor of one half has been included with curvature, this would quarter the curvature energy compared to the real energy, due to the squared curvature. The bending modulus used (ca. 5 kcal/mol) is small on the scale of typically observed biological bending moduli. This suggests the curvature energies are indeed much higher even than the high values reported. Some of this may be due to the spontaneous curvature of the lipids and perhaps the effect of the protein modifying the nearby lipids properties.
* It is unclear how CDL is supporting SC formation if its effect stabilizing the membrane deformation is strong or if it is acting as an electrostatic glue. While this is a weakenss for a definite quantification of the effect of CDL on SC formation, the study presents an interesting observation of CDL redistribution and could be an interesting topic for future work.
In summary, the qualitative data presented are interesting (especially the combination of molecular modeling with simpler Monte Carlo modeling aiding broader interpretation of the results). The energies of the membrane deformations are quite large. This might reflect the roles of specific lipids stabilizing those deformations, or the inherent difficulty in characterizing nanometer-scale curvature.
-

Reviewer #3 (Public review):
Summary:
In this contribution, the authors report atomistic, coarse-grained and lattice simulations to analyze the mechanism of supercomplex (SC) formation in mitochondria. The results highlight the importance of membrane deformation as one of the major driving forces for the SC formation, which is not entirely surprising given prior work on membrane protein assembly, but certainly of major mechanistic significance for the specific systems of interest.
Strengths:
The combination of complementary approaches, including an interesting (re)analysis of cryo-EM data, is particularly powerful, and might be applicable to the analysis of related systems. The calculations also revealed that SC formation has interesting impacts on the structural and dynamical (motional correlation) properties of the individual protein …
Reviewer #3 (Public review):
Summary:
In this contribution, the authors report atomistic, coarse-grained and lattice simulations to analyze the mechanism of supercomplex (SC) formation in mitochondria. The results highlight the importance of membrane deformation as one of the major driving forces for the SC formation, which is not entirely surprising given prior work on membrane protein assembly, but certainly of major mechanistic significance for the specific systems of interest.
Strengths:
The combination of complementary approaches, including an interesting (re)analysis of cryo-EM data, is particularly powerful, and might be applicable to the analysis of related systems. The calculations also revealed that SC formation has interesting impacts on the structural and dynamical (motional correlation) properties of the individual protein components, suggesting further functional relevance of SC formation. In the revision, the authors further clarified and quantified their analysis of membrane responses, leading to further insights into membrane contributions. They have also toned down the decomposition of membrane contributions into enthalpic and entropic contributions, which is difficult to do. Overall, the study is rather thorough, highly creative and the impact on the field is expected to be significant.
Weaknesses:
Upon revision, I believe the weakness identified in previous work has been largely alleviated.
-

Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written but identified a number of technical issues that I suggest should be addressed:
We thank Reviewer 1 for finding our work interesting. We have addressed the technical issues below.
(1) Neither the acyl chain chemical makeup nor the protonation state of CDL are specified. The acyl chain is likely 18:2/18:2/18:2/18:2, but the choice of the protonation state is not straightforward.
We thank the Reviewer for highlighting this missing information. We have now added this …
Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written but identified a number of technical issues that I suggest should be addressed:
We thank Reviewer 1 for finding our work interesting. We have addressed the technical issues below.
(1) Neither the acyl chain chemical makeup nor the protonation state of CDL are specified. The acyl chain is likely 18:2/18:2/18:2/18:2, but the choice of the protonation state is not straightforward.
We thank the Reviewer for highlighting this missing information. We have now added this information in the Materials and Methods section:
"…were performed in a POPC:POPE:cardiolipin (2:2:1) membrane containing 5 mol% QH2 / Q (1:1 ratio). Cardiolipin was modeled as tetraoleoyl cardiolipin (18:1/18:1/18:1/18:1) with a headgroup modeled in a singly protonated state (with Qtot=-1)."
(2) The analysis of the bilayer deformation lacks membrane mechanical expertise. Here I am not ridiculing the authors - the presentation is very conservative: they find a deformed bilayer, do not say what the energy is, but rather try a range of energies in their Monte Carlo model - a good strategy for a group that focuses on protein simulations. The bending modulus and area compressibility modulus are part of the standard model for quantifying the energy of a deformed membrane. I suppose in theory these might be computed by looking at the per-lipid distribution in thickness fluctuations, but this route is extremely perilous on a per-molecule basis. Instead, the fluctuation in the projected area of a lipid patch is used to imply the modulus [see Venable et al "Mechanical properties of lipid bilayers from molecular dynamics simulation" 2015 and citations within]. Variations in the local thickness of the membrane imply local variations of the leaflet normal vector (the vector perpendicular to the leaflet surface), which is curvature. With curvature and thickness, the deformation energy is analyzed.
See:
Two papers: "Gramicidin A Channel Formation Induces Local Lipid Redistribution" by Olaf Andersen and colleagues. Here the formation of a short peptide dimer is experimentally linked to hydrophobic mismatch. The presence of a short lipid reduces the influence of the mismatch. See below regarding their model cardiolipin, which they claim is shorter than the surrounding lipid matrix.
Also, see:
Faraldo-Gomez lab "Membrane transporter dimerization driven by differential lipid solvation energetics of dissociated and associated states", 2021. Mondal et al "Membrane Driven Spatial Organization of GPCRs" 2013 and many citations within these papers.
While I strongly recommend putting the membrane deformation into standard model terms, I believe the authors should retain the basic conservative approach that the membrane is strongly deformed around the proteins and that making the SC reduces the deformation, then exploring the consequences with their discrete model.
We thank the Reviewer for the suggestions and for pointing out the additional references, which are now cited in the revised manuscript. The analysis is indeed significantly more complex for large multi-million atom supercomplexes in comparison to small peptides (gramicidin A) or model systems of lipid membranes. However, in the revised manuscript, we have conducted further analysis on the membrane curvature effects based on the suggestions. We were able to estimate the energetic contribution of the changes in local membrane thickness and curvature, which are now summarized in Table 1, and described in the main text and SI. We find that both the curvature and local thickness contribute to the increased stability of SC.
We have now extensively modified the result to differentiate between different components of membrane strain properly:
"We observe a local decrease in the membrane thickness at the protein-lipid interface (Fig. 2G, Fig S2A,D,E), likely arising from the thinner hydrophobic belt region of the OXPHOS proteins (ca. 30 Å, Fig. S1A) relative to the lipid membrane (40.5 Å, Fig. S1). We further observe ∼30% accumulation of cardiolipin at the thinner hydrophobic belt regions (Fig. 2H, Fig. S2B,F,G), with an inhomogeneous distribution around the OXPHOS complexes. While specific interactions between CDL and protein residues may contribute to this enrichment (Fig. 2N), CDL prefers thermodynamically thinner membranes (∼38 Å, Fig. S1B, Fig. S5F). These changes are further reflected in the reduced end-toend distance of lipid chains in the local membrane belt (see Methods, Fig. S6, cf. also Refs. (41-44). In addition to the perturbations in the local membrane thickness, the OXPHOS proteins also induce a subtle inward curvature towards the protein-lipid interface (Fig. S5G), which could modulate the accessibility of the Q/QH2 substrate into the active sites of CI and CIII2 (see below, section Discussion). This curvature is accompanied by a distortion of the local membrane plane itself (Fig. 2A-F, Fig. S4AC, Fig. S7), with perpendicular leaflet displacements reaching up to ~2 nm relative to the average leaflet plane.
To quantify the membrane strain effects, we analyzed the cgMD trajectories by projecting the membrane surface onto a 2-dimensional grid and calculating the local membrane height and thickness at each grid point. From these values, we quantified the local membrane curvature (Fig. S5H), which measures the energetic cost of deforming the membrane from a flat geometry (ΔGcurv). We also computed the energetics associated with changes in the membrane thickness, assessed from the deviations from an ideal local membrane in the absence of embedded proteins (ΔGthick, see Supporting Information, for technical details). Our analysis suggests that both contributions are substantially reduced upon formation of the SC, with the curvature decreasing by 19.8 ± 1.3 kcal mol-1 and the thickness penalty by 2.8 ± 2.0 kcal mol-1 (Table 1). These results indicate a significant thermodynamic advantage for SC formation, as it minimizes lipid deformation and stabilizes the membrane environment surrounding Complex I and III.”
[…]
“Taken together, the analysis suggests that the OXPHOS complexes affect the mechanical properties of the membranes by inducing a small inwards curvature towards the protein-lipid interface (Fig. S5), resulting in a membrane deformation effect, while the SC formation releases some deformation energy relative to the isolated OXPHOS complexes. The localization of specific lipids around the membrane proteins, as well as local membrane perturbation effects, is also supported by simulations of other membrane proteins (45, 46), suggesting that the effects could arise from general protein-membrane interactions.”
Our Supporting Information section now provides additional information about the membrane curvature.
(41) R. M. Venable, F. L. H. Brown, R. W. Pastor, Mechanical properties of lipid bilayers from molecular dynamics simulation. Chemistry and Physics of Lipids 192, 60-74 (2015).
(42) R. Chadda et al., Membrane transporter dimerization driven by differential lipid solvation energetics of dissociated and associated states. eLife 10, e63288 (2021).
(43) S. Mondal et al., Membrane Driven Spatial Organization of GPCRs. Scientific Reports 3, 2909 (2013).
(44) J. A. Lundbæk, S. A. Collingwood, H. I. Ingólfsson, R. Kapoor, O. S. Andersen, Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. Journal of The Royal Society Interface 7, 373-395 (2009).
We also expanded our SI Method section to account for the new calculations:
“Analysis of lipid chain end-to-end length
To probe the protein-induced deformation effect of the membrane, the membrane curvature (H), and the end-to-end distance between the lipid chains, were computed based on aMD and cgMD simulations. The lipid chain length was computed from simulations A1-A6 and C1 based on the first and last carbon atoms of each lipid chain. For example, the end-to-end length of a cardiolipin chain was determined as the distance between atom “CA1” and atom “CA18”.
“Membrane Curvature and Deformation Energy
The local mean curvature of the membrane midplane was computed by approximating the membrane surface as a height function Z(x,y), defined as the average location of the N-side and P-side leaflets at each grid point. Based on this, the mean curvature H(x,y) was calculated as,
where the derivatives are defined as
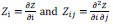 .
.The thickness deformation energy was computed from the local thickness d(x,y) relative to a reference thickness distribution F(d), derived from membrane-only simulations, and converted to a free energy profile via Boltzmann inversion. At each grid point, the F(d) was summed over the grid,
The bending deformation energy was computed from the mean curvature field H(x,y), assuming a constant bilayer bending modulus κ (taken as 20 kJ mol-1 = 4.78 kcal mol-1):
where ΔA is the area of the grid cell.
The thickness and curvature fields were obtained by projecting the coarse-grained MD trajectories (one frame per ns) onto a 2D-grid with a resolution of 0.5 nm. Grid points with low occupancy were downweighted to mitigate noise. More specifically, points with counts below 50% of the median grid count were scaled linearly by their relative count value. To focus the analysis on the region around the protein– membrane interface, only grid points within a radius of 20 nm from the center of the complex were included in the energy calculations. Energies were normalized to an effective membrane area of 1000 nm2 to facilitate the comparison between systems. Bootstrapping with resampling over frames was performed to estimate the standard deviations of Gthick and Gcurv.
We find that Gcurve converges slowly due to its sensitivity to local derivatives and the small grid size required to resolve the curvature contribution near the protein. Consequently, tens of microseconds of simulations were necessary to obtain well-converged estimates of the curvature energy.”
(1) If CDL matches the hydrophobic thickness of the protein it would disrupt SC formation, not favor it. The authors' hypothesis is that the SC stabilizes the deformed membrane around the separated elements. Lipids that are compatible with the monomer deformed region stabilize the monomer, similarly to a surfactant. That is, if CDL prefers the interface because the interface is thin and their CDL is thin, CDL should prevent SC formation. A simpler hypothesis is that CDL's unique electrostatics are part of the glue.
We rephrased the corresponding paragraph in the Discussion section to reflect the role of electrostatics for the behavior of cardiolipin.
"…supporting the involvement of CDL as a "SC glue". In this regard, electrostatic effects arising from the negatively charged cardiolipin headgroup could play an important role in the interaction of the OXPHOS complexes."
Generally our simulations suggest that CDL prefers thinner membranes, which could rationalize these findings.
"We find that CDL prefers thinner membranes relative to the neutral phospholipids (PE/PC, Fig. S5F),[…]”
(2) Error bars for lipid and Q* enrichments should be computed averaging over multi-lipid regions of the protein interface, e.g., dividing the protein-lipid interface into six to ten domains, in particular functionally relevant regions. Anionic lipids may have long, >500 ns residence times, which makes lipid enrichment large and characterization of error bars challenging in short simulations. Smaller regions will be noisy. The plots depicted in, for example, Figure S2 are noisy.
It is indeed challenging to capture lipid movements on the timescales accessible for atomistic MD, and hence the data in Figure S2 contains some noise. In this regard, for the cgMD data presented in the revised Fig. S2H,I, the concentration data was averaged for six domains of the protein-lipid interface.
(3) The membrane deformation is repeatedly referred to as "entropic" without justification. The bilayer has significant entropic and enthalpic terms just like any biomolecule, why are the authors singling out entropy? The standard "Helfrich" energetic Hamiltonian is a free energy model in that it implicitly integrates over many lipid degrees of freedom.
We apologize for the unclear message – our intention was not to claim that the effects are purely entropic, but could arise from a combination of both entropic and enthalpic effects. We hope that this has now been better clarified in the revised manuscript. We also agree that it is difficult to separate between entropic and enthalpic effects. However, we wish to point out that, e.g., the temperature-dependence of the SC formation suggests that the entropic contribution is also affecting the process.
Regarding the Helfrich Hamiltonian, we note that the standard model assumes a homogeneous fluid-like sheet. We have thus difficulties in relating this model to capture the local effects.
Revisions / clarifications in the main manuscript:
"SC formation is affected by both enthalpic and entropic effects."
"We have shown here that the respiratory chain complexes perturb the IMM by affecting the local membrane dynamics. The perturbed thickness and alteration in the lipid dynamics lead to an energetic penalty, which can be related to molecular strain effects, as suggested by the changes of both the internal energy of lipid and their interaction with the surroundings (Fig. S2, S5, S6), which are likely to be of enthalpic origin. However, lipid binding to the OXPHOS complex also results in a reduction in the translational and rotational motion of the lipids and quinone (Fig. S8-S9), which could result in entropic changes. The strain effects are therefore likely to arise from a combination of enthalpic and entropic effects."
(4) Figure S7 shows the surface area per lipid and leaflet height. This appears to show a result that is central to the interpretation of SC formation but which makes very little sense. One simply does not increase both the height and area of a lipid. This is a change in the lipid volume! The bulk compressibility of most anything is much higher than its Young's modulus [similar to area compressibility]. Instead, something else has happened. My guess is that there is *bilayer* curvature around these proteins and that it has been misinterpreted as area/thickness changes with opposite signs of the two leaflets. If a leaflet gets thin, its area expands. If the manuscript had more details regarding how they computed thickness I could help more. Perhaps they measured the height of a specific atom of the lipid above the average mid-plane normal? The mid-plane of a highly curved membrane would deflect from zero locally and could be misinterpreted as a thickness change.
We thank the Reviewer for this insightful comment. We chose to define the membrane thickness based on the height of the lipid P-atoms above the average midplane normal. The Reviewer is correct that this measurement gives a changing thickness for a highly curved membrane. However, in this scenario, the thickness would always be overestimated [dtrue = dmeasured / cos (angle between global mid-plane normal and local mid-plane normal)]. Therefore, since we observe a smaller thickness at the protein-lipid interface, the effect is not likely to result from an artifact. For further clarification, see Fig. S4I showing the averaged local position of the Patoms in the cgMD simulations, which further supports that there is a local deformation of the lipid.
The changes in the local membrane thickness are also supported by our analysis of the membrane thickness (Fig.S2A) and by the lipid chain length distributions (Fig.S6).
(5) The authors write expertly about how conformational changes are interpreted in terms of function but the language is repeatedly suggestive. Can they put their findings into a more quantitative form with statistical analysis? "The EDA thus suggests that the dynamics of CI and CIII2 are allosterically coupled."
We extended our analysis on the allosteric effects, which is now described in the revised main text, the SI and the Methods section:
"In this regard, our graph theoretical analysis (Fig. S11C,D) further indicates that ligand binding to Complex I induces a dynamic crosstalk between NDUFA5 and NDUFA10, consistent with previous work (50, 51), and affecting also the motion of UQCRC2 with respect to its surroundings. Taken together, these effects suggest that the dynamics of CI and CIII2 show some correlation that could result in allosteric effects, as also indicated based on cryo-EM analysis (40)."
“Extended Methods
Allosteric Network Analysis. Interactions between amino acid residues were modeled as an interaction graph, where each residue was represented by a vertex. Two nodes were connected by an edge, if the Ca atoms of the corresponding amino acid residues were closer than 7.5 Å for more than 50% of the frames of simulations S1-S6 (time step of frames: 1 ns). (7) This analysis was carried out for the aMD simulations of the supercomplex, analyzing differences between the Q bound and apo states (simulations A1+A2+A3 vs. A4+A5+A6).”
(6) The authors write "We find that an increase in the lipid tail length decreases the relative stability of the SC (Figure S5C)" This is a critical point but I could not interpret Figure S5C consistently with this sentence. Can the authors explain this?
We apologize for this oversight. This sentence should refer to Fig. S5F, which has now been corrected. We have additionally updated the figure to provide an improved estimation of the thickness contribution based on the lipid tail length.
"We find that an increase in the lipid tail length decreases the relative stability of the SC (Fig. S5F)"
(7) The authors use a 6x6 and 15x15 lattice to analyze SC formation. The SC assembly has 6 units of E_strain favoring its assembly, which they take up to 4 kT. At 3 kT, the SC should be favored by 18 kT, or a Boltzmann factor of 10^8. With only 225 sites, specific and non-specific complex formation should be robust. Can the authors please check their numbers or provide a qualitative guide to the data that would make clear what I'm missing?
In the revised manuscript, we have now clarified the definition of the lattice model and the respective energies:
In summary, the qualitative data presented are interesting (especially the combination of molecular modeling with simpler Monte Carlo modeling aiding broader interpretation of the results) ... but confusing in terms of the non-standard presentation of membrane mechanics and the difficulty of this reviewer to interpret some of the underlying figures: especially, the thickness of the leaflets around the protein and the relative thickness of cardiolipin. Resolving the quantitative interpretation of the bilayer deformation would greatly enhance the significance of their Monte Carlo model of SC formation.
We thank the Reviewer for the helpful suggestion. We hope that the revisions help to clarify the non-standard presentation and connect to concepts used in the lipid membrane community.
Reviewer #2 (Public review):
Summary:
The authors have used large-scale atomistic and coarse-grained molecular dynamics simulations on the respiratory chain complex and investigated the effect of the complex on the inner mitochondrial membrane. They have also used a simple phenomenological model to establish that the super complex (SC) assembly and stabilisation are driven by the interplay between the "entropic" forces due to strain energy and the enthalpies forces (specific and non-specific) between lipid and protein domains. The authors also show that the SC in the membrane leads to thinning and there is preferential localisation of certain lipids (Cardiolipin) in the annular region of the complex. The data reports that the SC assembly has an effect on the conformational dynamics of individual proteins making up the assembled complex and they undergo "allosteric crosstalk" to maintain the stable functional complex. From their conformational analyses of the proteins (individual and while in the complex) and membrane "structural" properties (such as thinning/lateral organization etc) as well from the out of their phenomenological lattice model, the authors have provided possible implications and molecular origin about the function of the complex in terms of aspects such as charge currents in internal mitochondrion membrane, proton transport activity and ATP synthesis.
Strengths:
The work is bold in terms of undertaking modelling and simulation of such a large complex that requires simulations of about a million atoms for long time scales. This requires technical acumen and resources. Also, the effort to make connections to experimental readouts has to be appreciated (though it is difficult to connect functional pathways with limited (additive forcefield) simulations.
We thank the Reviewer for recognizing the challenge in simulating multimillion atom membrane proteins. We also thank the Reviewer for recognizing the connections we have made to different experiments. Our work indeed relies on atomistic and coarse-grained molecular simulations, which are widely recognized to provide accurate models of membrane proteins.
Weakness:
There are several weaknesses in the paper (please see the list below). Claims such as "entropic effect", "membrane strain energy" and "allosteric cross talks" are not properly supported by evidence and seem far-fetched at times. There are other weaknesses as well. Please see the list below.
We thank the Reviewer for pointing out that key concepts needed further clarification. Please see answers to specific questions below:
(i) Membrane "strain energy" has been loosely used and no effort is made to explain what the authors mean by the term and how they would quantify it. If the membrane is simulated in stress-free conditions, where are strains building up from?
We thank the Reviewer for this important question. In the revised manuscript, we have toned down the assignment of the effects into pure entropic or enthalpic effects. We have also provided further clarification of the effects observed in the membrane.
Example of revisions / clarifications in the main text:
"SC formation is affected by both enthalpic and entropic effects."
"We have shown here that the respiratory chain complexes perturb the IMM by affecting the local membrane dynamics. The perturbed thickness and alteration in the lipid dynamics lead to an energetic penalty, which can be related to molecular strain effects, as suggested by the changes of both the internal energy of lipid and their interaction with the surroundings (Fig. S2, S5, S6), which are likely to be of enthalpic origin. However, lipid binding to the OXPHOS complex, also results in a reduction in the translational and rotational motion of the lipids and quinone (Fig. S8-S9), which could result in entropic changes. The strain effects are therefore likely to arise from a combination of enthalpic and entropic effects."
We have also revised the result section, where we now have explicitly defined and clarified the different contributions to membrane strain, observed in our simulations:
In the following, we define membrane strain as the local perturbations of the lipid bilayer induced by protein-membrane interactions. These include changes in (i) membrane thickness, (ii) the local membrane composition, (iii) lipid chain configurations, and (iv) local curvature of the membrane plane relative to an undisturbed, protein-free bilayer. Together, these phenomena reflect the thermodynamic effects associated with accommodating large protein complexes within the membrane.
We now also provide a more quantitative estimation of the membrane strain based on the contribution of changes in local thickness and curvature, summarize in Table 1.
(ii) In result #1 (Protein membrane interaction modulates the lipid dynamics ....), I strongly feel that the readouts from simulations are overinterpreted. Membrane lateral organization in terms of lipids having preferential localisation is not new (see doi: 10.1021/acscentsci.8b00143) nor membrane thinning and implications to function (https://doi.org/10.1091/mbc.E20-12-0794). The distortions that are visible could be due to a mismatch in the number of lipids that need to be there between the upper and lower leaflets after the protein complex is incorporated. Also, the physiological membrane will have several chemically different lipids that will minimise such distortions as well as would be asymmetric across the leaflets - none of which has been considered. Connecting chain length to strain energy is also not well supported - are the authors trying to correlate membrane order (Lo vs Ld) with strain energy?
We thank the Reviewer for the suggestions. The role of the membrane in driving supercomplex formation has not, to our knowledge, been suggested before. There are certainly many important studies, which have been better highlighted in the revised manuscript. In this context, we also now cite the papers Srivastava & coworkers and Tielemann & coworkers.
“The localization of specific lipids around the membrane proteins, as well as local membrane perturbation effects, are also supported by simulations of other membrane proteins (45, 46), suggesting that the effects could arise from general protein-membrane interactions.”
(45) V. Corradi et al., Lipid–Protein Interactions Are Unique Fingerprints for Membrane Proteins. ACS Central Science 4 (June 13, 2018).
(46) K. Baratam, K. Jha, A. Srivastava, Flexible pivoting of dynamin pleckstrin homology domain catalyzes fission: insights into molecular degrees of freedom. Molecular Biology of the Cell 32 (2021 Jul 1).
Physiological membrane will have several chemically different lipids that will minimise such distortions as well as would be asymmetric across the leaflets
We agree with this point. As shown in Figs. 2H,N, S6, S13, we suggest that cardiolipin functions as a buffer molecule. However, very little is experimentally known about the asymmetric distribution of lipids in the IMM. Therefore, modelling the effect of asymmetry across the left is outside the scope of this study. Moreover, as now better clarified in the revised manuscript, we agree that it is difficult to unambiguously divide the effect into enthalpic and entropic contributions.
To address the main concern of the Reviewer, we have updated the main text and Supporting Information to clearly state the different aspects of how the proteinmembrane interactions induce perturbations of the lipid bilayer. We define these effects as membrane strain. We now use the changes in local thickness and local curvature to quantify the effect of membrane strain on the stability of the respiratory SC.
(iii) Entropic effect: What is the evidence towards the entropic effect? If strain energy is entropic, the authors first need to establish that. They discuss enthalpy-entropy compensation but there is no clear data or evidence to support that argument. The lipids will rearrange themselves or have a preference to be close to certain regions of the protein and that generally arises because of enthalpies reasons (see the body of work done by Carol Robinson with Mass Spec where certain lipids prefer proteins in the GAS phase, certainly there is no entropy at play there). I find the claims of entropic effects very unconvincing.
We agree that it is difficult to distinguish the entropic vs. enthalpic contributions. In the revised manuscript, we better clarify that both effects are likely to be involved.
The native MS work by Robinson and coworkers and others support that many lipids are strongly bound to membrane proteins, as also supported by the local binding of certain lipid molecules, such as CDL to the SC (Figs. S2, S6, S13).
We suggest that the accumulation of cardiolipin at the protein-lipid interface involves a combination of entropic and enthalpic effects, arising from the reduction of the lipid mobility (entropy) as indicated by lowered diffusion (Fig. S9), and formation of noncovalent bonds between the lipid and the OXPHOS protein (Fig. S14).
We added further clarification to the Discussion section.
“Taken together, our combined findings suggest that the SC formation is affected by thermodynamic effects that reduce the molecular strain in the lipid membrane, whilst the perturbed micro-environment also affects the lipid and Q dynamics, as well as the dynamics of the OXPHOS proteins (see below).”
(iv) The changes in conformations dynamics are subtle as reported by the authors and the allosteric arguments are made based on normal mode analyses. In the complex, there are large overlapping regions between the CI, CIII2, and SCI/III2. I am not sure how the allosteric crosstalk claim is established in this work - some more analyses and data would be useful. Normal mode analyses (EDA) suggest that the motions are coupled and correlated - I am not convinced that it suggests that there is allosteric cross-talk.
Our analysis suggests that the SC changes the dynamics of the system. Although it is difficult to assign how these effects result in activity modulation of the system, we note these changes relate to sites that are central for the charge transfer reactions. We thank the Reviewer for suggesting to extend the analysis, which further suggests that regions of the proteins could be allosterically coupled.
(v) The lattice model should be described better and the rationale for choosing the equation needs to be established. Specific interactions look unfavourable in the equation as compared to non-specific interactions.
We have now provided further clarification of the lattice model in the Methods section. Addition to the main text:
“Lattice model of SC formation. A lattice model of the CI and CIII2 was constructed (Fig. 4A,B) by modeling the OXPHOS proteins in unique grid positions on a 2D N×N lattice. Depending on the relative orientation, the protein-protein interaction was described by specific interactions (giving rise to the energetic contribution Especific < 0) and non-specific interactions (Enon-specific > 0). The membrane-protein interaction determined the strain energy of the membrane (Estrain), based on the number of neighboring "lipid" occupied grids that are in contact with proteins (Fig. 4A). The interaction between the lipids was indirectly accounted for by the background energy of the model. The proteins could occupy four unique orientations on a grid ([North, East, South, West]). The states and their respective energies that the system can visit are summarized in Table S6.”
“The conformational landscape was sampled by Monte Carlo (MC) using 107 MC iterations with 100 replicas. Temperature effects were modeled by varying β, and the effect of different protein-to-lipid ratios by increasing the grid area. The simulation details can be found in Table S7.”
Reviewer #3 (Public review):
Summary:
In this contribution, the authors report atomistic, coarse-grained, and lattice simulations to analyze the mechanism of supercomplex (SC) formation in mitochondria. The results highlight the importance of membrane deformation as one of the major driving forces for SC formation, which is not entirely surprising given prior work on membrane protein assembly, but certainly of major mechanistic significance for the specific systems of interest.
Strengths:
The combination of complementary approaches, including an interesting (re)analysis of cryo-EM data, is particularly powerful and might be applicable to the analysis of related systems. The calculations also revealed that SC formation has interesting impacts on the structural and dynamical (motional correlation) properties of the individual protein components, suggesting further functional relevance of SC formation. Overall, the study is rather thorough and highly creative, and the impact on the field is expected to be significant.
Weaknesses:
In general, I don't think the work contains any obvious weaknesses, although I was left with some questions.
We thank the Reviewer for acknowledging that our work is thorough and creative, and that it is likely to have a significant impact on the field.
Recommendations for the authors:
Reviewer #1 (Recommendations for the authors):
Diffusion is quantified in speed units (Figure S8). The authors should explain why they have used an apparently incorrect model for quantifying diffusion. The variance of the distribution of a diffusing molecule is linear with time, not its standard deviation (as I suppose I would use for computing effective molecular speed). Perhaps they are quantifying residence times, in which molecules near a wall (protein) will appear to have half the movements of a bulk molecule. This is confusing.
We thank the Reviewer for the comment. The data shown in previous version of Figure S8 corresponded to the effective molecular velocity, which is now clarified in the revised figure (now Fig. S9). This measure was used to reflect the average residence time of the groups in the vicinity of the sites.
However, as suggested by the Reviewer, we now also analyzed the positiondependent diffusion of the quinone in the new Figure S9:
(2) With a highly charged bilayer a large water layer is necessary to verify that the concentration of salt is plateauing at 150 mM at the box edge. 45 A appears to be the default in CHARMM-GUI, but this default guidance is not based on the charge of the bilayer. I suggest the authors plot the average concentration of both anions and cations in mM units along the z coordinate of the simulation cell.
We thank the Reviewer for the suggestion. We have now provided an analysis of the average ion concentrations along the z coordinate, supporting that the salt concentration plateaus at 150 mM at the box edge.
Typos:
SI: "POPC/POPE or CLD" should be CDL
We apologize for the mistake. We have corrected the typos:
"of the membrane thickness in a POPC/POPE/CDL/QH2 membrane and a CDL membrane."
"a pure CDL membrane"
Reviewer #2 (Recommendations for the authors):
(1) Suggestion regarding membrane strain energy claims:
Changes in area per lipid and membrane thinning are surely not akin to membrane strain energy changes. At best, the authors should calculate the area compressibility (both in bilayers with and without proteins) and then make comments. In general, if they are talking about the in-plane properties (bilayer being liquid in 2D), I do not see how they can discuss membrane strain energy with NPT=1 atms barostat reservoir that they are simulating against. At least they can try to plot the membrane lateral pressures in various conditions and then start making such comments. If it was a closed vesicle, I would expect some tension in the membrane due to the closed surface but in the conditions in which the simulations are run, I do not see how strain is so important. If the authors want to be more rigorous, they can calculate "atomic viral" values by doing a tessellation and showing the data to make their point. Strain energy would mean that there is a modulus in-plane. Bending modulus would surely change with membrane thinning and area compressibility changes (simple plate theory) but linear strain is surely something to be defined well before making claims out of it.
Our work shows that the OXPHOS proteins alter the local membrane thickness and curvature, and we now quantify the deformation penalty associated with that (Table 1). As stated above, we now provide a better definition and description 'membrane strain’ and the observed effect, which is likely to contain both enthalpic and entropic contributions.
As suggested by the Reviewer, we have computed the lateral pressure profiles around the OXPHOS proteins, further supporting that there are energetic effects related to the "solvation" of the membrane proteins in the IMM. To this end, Figs. S2D,E; Figure S4I and Fig. S5G,H shows the membrane distortion effect; while in Fig. S5A supports that there the 'internal energy' of the lipids changes as result of the SC formation, further justifying that these effects can be assigned as 'strain effects'. The analysis has also been extended by computing the end-to-end distances, shown in Fig. S6.
Unfortunately, it is technically unfeasible to accurately estimate the area compressibility, bending modulus, or the atomic virial for the present multi-million membrane protein simulations.
Summary of Revisions/Additions:
Fig. S2 [...] (D, E) Difference in the membrane thickness around the SC relative to CI (left) or relative to CIII2 (right) from (D) aMD and (E) cgMD.
Fig. S4. [...] (I) Visualization of the membrane distortion effect.
Fig. S5. Analysis of membrane-induced distortion effects. (A) Relative strain effect relative to a lipid membrane from atomistic MD simulations of the SCI/III2, CI, and CIII2, suggesting reduction of the membrane strain (blue patches) in the SC surroundings. The figure shows the non-bonded energies relative to the average non-bonded energies from membrane simulations (simulation M4, Table S1). (B) The lipid strain contribution for different lipids calculated from non-bonded interaction energies of the lipids relative to the average lipid interaction in a IMM membrane model (simulation M4). The figure shows the relative strain contribution for nearby lipids (r < 2 Å, in color from panel (C), and lipids >5 Å from the OXPHOS proteins. (C) Selection of lipids (< 2 Å) interacting with the OXPHOS proteins. (D) Potential of mean force (PMF) of membrane thickness derived from thickness distributions from cgMD simulations of a membrane, the SCI/III2, CI, and CIII2. (E) Membrane thickness as a function of CDL concentration from cgMD simulations. (F) ΔGthick of the SC as a function of membrane thickness based on cgMD simulations. (G) Membrane curvature around the SCI/III2 (left), CI (middle), and CIII2 (right) from atomistic simulations. (H) Squared membrane curvature obtained from cgMD simulations, within a 20 nm radius around the center of the system. These maps correspond to the curvature field used in the calculation of the bending deformation energy term (Gcurv).
Fig. S6. Analysis of lipid end-to-end distance from aMD simulations of (A) SC, (B) CI, (C) CIII2.
(2) Membrane distortions:
Membrane distortions can arise due to a mismatch in the area between the upper leaflet and the lower left especially when a protein is embedded. Authors can carefully choose the numbers to keep the membrane stable.
We have further clarified in the revised manuscript that the membranes are stable in all simulation setups. During building the simulation setups, it was carefully considered that no leaflet introduced higher lipid densities that could result in artificial displacements. Our results of the local changes in the lipid dynamics and structure around the OXPHOS complexes are independently supported by both our atomistic and coarse-grained simulations, which contain significantly larger membranes. Moreover, as discussed in our work, the local membrane distortion is also experimentally supported by cryoEM analysis as well as recent in situ cryoTEM data, showing that the OXPHOS proteins indeed affect the local membrane properties.
Clarifications/Additions to the main text:
“We find that the individual OXPHOS complexes, CI and CIII2, induce pronounced membrane strain effects, supported both by our aMD (Fig. S2A) and cgMD simulations with a large surrounding membrane (Fig. 2G).“
” The localization of specific lipids around the membrane proteins, as well as local membrane perturbation effects, are also supported by simulations of other membrane proteins (45, 46), suggesting that the effects could arise from general protein-membrane interactions.”
"During construction of the simulation setups, it was carefully considered that no leaflet introduced higher lipid densities that could result in artificial displacement effects."
(3) Strain energy as an entropic effect:
Please establish that the strain energy (if at all present) can be called an entropic effect.
We have now better clarified that the SC formation results from combined enthalpic and entropic effects. We apologize that the previous version of the text was unclear in this respect.
To further probe the involvement of entropic effects, we derived entropic and enthalpic contributions from our lattice model. The model supports that increased strain contributions also alters the entropic contributions, further supporting the coupling between the effects.
We have also clarified our definition of the effects:
" The perturbed thickness and alteration in the lipid dynamics leads to an energetic penalty, which can be related to molecular strain effects, as suggested by the changes of both the internal energy of lipid and their interaction with the surroundings (Fig. S2, S5, S6), which are likely to be of enthalpic origin. However, lipid binding to the OXPHOS complex, also results in a reduction in the translational and rotational motion of the lipids and quinone (Fig. S8-S9), which could result in entropic changes. The strain effects are therefore likely to arise from a combination of enthalpic and entropic effects."
(4) Allosteric cross-talk:
A thorough network analysis (looking at aspects like graph laplacian, edge weights, eigenvector centrality, changes in characteristic path length, etc can be undertaken to establish allostery (see https://doi.org/10.1093/glycob/cwad094, Ruth Nussinov/Ivet Bahar papers).
We have expanded the network analysis as suggested by the Reviewer. In this regard, we have expanded the analysis by computing the covariance matrix, further supporting that the SC could involve correlated protein dynamics. We observe a prominent change especially with respect to the ligand state of Complex I, indicative of some degree of allostery, while we find that the apo state of Complex I leads to a slight uncoupling of the motion between CI and CIII2.
Additions in the main text:
In this regard, our graph theoretical analysis (Fig. S11) further indicates that ligand binding to Complex I induces a dynamic crosstalk between NDUFA5 and NDUFA10, consistent with previous work (48, 49), and affecting also the motion of UQCRC2 with respect to its surroundings_._ Taken together, these effects suggest that the dynamics of CI and CIII2 show some correlation that could result in allosteric effects, as also indicated based on the cryoEM analysis.
(5) Lattice model:
The equation needs to be rationalised. For example, specific interaction (g_i g_j favours separation (lower energy when i and j are not next to each other), and nonspecific interaction favours proximity. Why is that? Also, the notation for degeneracy in partition function and the notation for lattice point. It is mentioned that the "interaction between the lipids was indirectly accounted for by the "background energy" of the model". If the packing/thinning etc are so important to the molecular simulations, will not the background energy change with changing lipid organising during complex formation?
We have further expanded the technical discussion of the energy terms in our lattice model.
For example, specific interaction (g_i g_j favours separation (lower energy when i and j are not next to each other), and non-specific interaction favours proximity. Why is that
"The gigj -term assigns a specific energy contribution when the OXPHOS complexes are in adjacent lattice points only in a correct orientation (modeling a specific non-covalent interaction between the complexes such as the Arg29FB4-Asp260C1/Glu259C1 interaction between CI and CIII2). The didj -term assigns a non-specific interaction for the OXPHOS complexes when they are in adjacent lattice points, but in a "wrong" orientation relative to each other to form a specific interaction. The
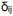 term introduces a strain into all lattice points surrounding an OXPHOS complex, mimicking the local membrane perturbation effects observed in our molecular simulations.
term introduces a strain into all lattice points surrounding an OXPHOS complex, mimicking the local membrane perturbation effects observed in our molecular simulations.This leads to the partition function,
where wi is the degeneracy of the state, modeling that the SC and OXPHOS proteins can reside at any lattice position of the membrane, and where β=1/kBT (kB, Boltzmann's constant; T, temperature). The probability of a given state i was calculated as,
with the free energy (G) defined as,
This discussion has been included in the methods sections to ensure that our work remains readable for the biological community studying supercomplexes from a biochemical, metabolic, and physiological perspectives.
(6) This is a minor issue but the paper is poorly organised and can be fixed readily. The figures are not referenced in order. For example, Figure 2G is discussed before discussing Figures 2A-2F (never discussed). Figure S2 is referenced before Figure S1.
Answer: We thank the Reviewer for pointing this out. The order of the figures was revised.
Reviewer #3 (Recommendations for the authors):
A few minor questions/suggestions, not necessarily in the order of importance:
(1) The discussion of the timescale of simulations is a bit misleading. For example, the discussion cites a timescale of 0.3 ms of CG simulations. The value is actually the sum of multiple CG simulations on the order of 50-75 microseconds. These are already very impressive lengths of CG simulations, there is no need to use the aggregated time to claim even longer time scales.
We thank the Reviewer for the suggestion on this important clarification. We have now modified the text and tables accordingly:
"(0.3 ms in cumulative simulation time, 50-75 μs/cgMD simulation)"
(2) The observation of cardiolipin (CDL) accumulation is interesting. How close are the head groups, relative to the electrostatic screening length at the interface? Should one worry about the potential change of protonation state coupled with the CDL redistribution?
Answer: We thank the Reviewer for this excellent comment, which has also been on our mind. The CDL indeed form contacts with various functional groups at the protein interface (as shown in Fig. S13), as well as bulk ions (sodium) that could tune the p_K_a of the CDLs, and result in a protonation change. We have clarified these effects in the revised manuscript:
"While CDL was modeled here in the singly anionic charged state (but cf. Fig. S5E), we note that the local electrostatic environment could tune their p_K_a that result in protonation changes of the lipid, consistent with its function as a proton collecting antenna (62)."
(3) The authors refer to the membrane strain effect as entropic. Since membrane bending implicates a free energy change that includes both enthalpic and entropic components, I wonder how the authors reached the conclusion that the effect is largely entropic in nature.
We agree with the Reviewer that the effects are likely to comprise both enthalpic and entropic contributions, which are difficult to separate in practice. To reflect this, we have now better clarified why we consider that both contributions are involved. We apologize that our previous version of the manuscript was unclear in this respect. Clarifications in the main text:
“The perturbed thickness and alteration in the lipid dynamics lead to an energetic penalty, which can be related to molecular strain effects, as suggested by the changes of both the internal energy of lipid and their interaction with the surroundings (Fig. S2, S5, S6), which are likely to be of enthalpic origin. However, lipid binding to the OXPHOS complex also results in a reduction in the translational and rotational motion of the lipids and quinone (Fig. S8-S9), which could result in entropic changes. The strain effects are therefore likely to arise from a combination of enthalpic and entropic effects."
(4) The authors refer to the computed dielectric constant as epsilon_perpendicular. Did the authors really distinguish the parallel and perpendicular component of the dielectric tensor, as was done by, for example, R. Netz and co-workers for planar surfaces?
We have extracted the perpendicular dielectric constant from the total dielectric profiles. We clarify that this differs from the formal definition of by Netz and coworkers.
“The calculations were performed by averaging the total M over fixed z values from the membrane plane. Note that this treatment differs from extraction of radial and axial contributions of the dielectric tensor, as developed by Netz and co-workers (cf. Ref. (3) and refs therein) that requires a more elaborate treatment, which is outside the scope of the present work.”
(3) P. Loche, C. Ayaz, A. Schlaich, Y. Uematsu, R.R. Netz. Giant Axial Dielectric Response in Water-Filled Nanotubes and Effective Electrostatic Ion-Ion Interactions from a Tensorial Dielectric Model. J Phys Chem B 123, 10850-10857 (2019).
(5) Regarding the effect of SC formation on protein structure and dynamics, especially allosteric effects, most of the discussions are rather qualitative in nature. More quantitative analysis would be valuable. For example, the authors did compute covariance matrix although it appears that they chose not to discuss the results in depth. Is the convergence of concern and therefore no thorough discussion is given?
We have now expanded the analysis by computing the covariance matrix, further supporting that the SC could involve correlated protein dynamics. We observe a prominent change, especially with respect to the ligand state of Complex I, indicative of some degree of allostery, while we find that the apo state of Complex I leads to a slight uncoupling of the motion between CI and CIII2.
Additions in the main text:
“In this regard, our graph theoretical analysis (Fig. S11) further indicates that ligand binding to Complex I induces a dynamic crosstalk between NDUFA5 and NDUFA10, consistent with previous work (48, 49), and affecting also the motion of UQCRC2 with respect to its surroundings. Taken together, these effects suggest that the dynamics of CI and CIII2 show some correlation that could result in allosteric effects, as also indicated based on the cryoEM analysis (40).”
(6) The discussion of quinone diffusion is interesting, although I'm a bit intrigued by the unit of the diffusion constant cited in the discussion. Perhaps a simple typo?
The plot showed the molecular velocity, which roughly corresponding to the residence times. However, as suggested by the Reviewer, we now also analyzed the position-dependent diffusion of the quinone in the new Figure S9:
-

eLife Assessment
In this potentially important study, the authors conducted extensive atomistic and coarse-grained simulations as well as a lattice Monte Carlo analysis to probe the driving force and functional impact of supercomplex formation in the inner mitochondrial membrane. The study highlighted the importance of membrane mechanics to the supercomplex formation and revealed differences in structural and dynamical features of the protein components upon complex formation. In its current form, the analysis is considered incomplete, especially concerning the contributions of membrane mechanics and allosteric coupling of key regions.
-

Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written but identified a number of technical issues that I suggest should be addressed:
(1) Neither the acyl chain chemical makeup nor the protonation state of CDL are specified. The acyl chain is likely 18:2/18:2/18:2/18:2, but the choice of the protonation state is not straightforward.
(2) The analysis of the bilayer deformation lacks membrane mechanical expertise. Here I am not ridiculing the authors - the presentation is very conservative: they find a deformed bilayer, do not say what the energy is, but rather try a range of energies in their Monte Carlo model - a good …
Reviewer #1 (Public review):
This paper by Poverlein et al reports the substantial membrane deformation around the oxidative phosphorylation super complex, proposing that this deformation is a key part of super complex formation. I found the paper interesting and well-written but identified a number of technical issues that I suggest should be addressed:
(1) Neither the acyl chain chemical makeup nor the protonation state of CDL are specified. The acyl chain is likely 18:2/18:2/18:2/18:2, but the choice of the protonation state is not straightforward.
(2) The analysis of the bilayer deformation lacks membrane mechanical expertise. Here I am not ridiculing the authors - the presentation is very conservative: they find a deformed bilayer, do not say what the energy is, but rather try a range of energies in their Monte Carlo model - a good strategy for a group that focuses on protein simulations. The bending modulus and area compressibility modulus are part of the standard model for quantifying the energy of a deformed membrane. I suppose in theory these might be computed by looking at the per-lipid distribution in thickness fluctuations, but this route is extremely perilous on a per-molecule basis. Instead, the fluctuation in the projected area of a lipid patch is used to imply the modulus [see Venable et al "Mechanical properties of lipid bilayers from molecular dynamics simulation" 2015 and citations within]. Variations in the local thickness of the membrane imply local variations of the leaflet normal vector (the vector perpendicular to the leaflet surface), which is curvature. With curvature and thickness, the deformation energy is analyzed.
See:
Two papers: "Gramicidin A Channel Formation Induces Local Lipid Redistribution" by Olaf Andersen and colleagues. Here the formation of a short peptide dimer is experimentally linked to hydrophobic mismatch. The presence of a short lipid reduces the influence of the mismatch. See below regarding their model cardiolipin, which they claim is shorter than the surrounding lipid matrix.Also, see:
Faraldo-Gomez lab "Membrane transporter dimerization driven by differential lipid solvation energetics of dissociated and associated states", 2021. Mondal et al "Membrane Driven Spatial Organization of GPCRs" 2013 and many citations within these papers.While I strongly recommend putting the membrane deformation into standard model terms, I believe the authors should retain the basic conservative approach that the membrane is strongly deformed around the proteins and that making the SC reduces the deformation, then exploring the consequences with their discrete model.
(1) If CDL matches the hydrophobic thickness of the protein it would disrupt SC formation, not favor it. The authors' hypothesis is that the SC stabilizes the deformed membrane around the separated elements. Lipids that are compatible with the monomer deformed region stabilize the monomer, similarly to a surfactant. That is, if CDL prefers the interface because the interface is thin and their CDL is thin, CDL should prevent SC formation. A simpler hypothesis is that CDL's unique electrostatics are part of the glue.
(2) Error bars for lipid and Q* enrichments should be computed averaging over multi-lipid regions of the protein interface, e.g., dividing the protein-lipid interface into six to ten domains, in particular functionally relevant regions. Anionic lipids may have long, >500 ns residence times, which makes lipid enrichment large and characterization of error bars challenging in short simulations. Smaller regions will be noisy. The plots depicted in, for example, Figure S2 are noisy.
(3) The membrane deformation is repeatedly referred to as "entropic" without justification. The bilayer has significant entropic and enthalpic terms just like any biomolecule, why are the authors singling out entropy? The standard "Helfrich" energetic Hamiltonian is a free energy model in that it implicitly integrates over many lipid degrees of freedom.
(4) Figure S7 shows the surface area per lipid and leaflet height. This appears to show a result that is central to the interpretation of SC formation but which makes very little sense. One simply does not increase both the height and area of a lipid. This is a change in the lipid volume! The bulk compressibility of most anything is much higher than its Young's modulus [similar to area compressibility]. Instead, something else has happened. My guess is that there is *bilayer* curvature around these proteins and that it has been misinterpreted as area/thickness changes with opposite signs of the two leaflets. If a leaflet gets thin, its area expands. If the manuscript had more details regarding how they computed thickness I could help more. Perhaps they measured the height of a specific atom of the lipid above the average mid-plane normal? The mid-plane of a highly curved membrane would deflect from zero locally and could be misinterpreted as a thickness change.
(5) The authors write expertly about how conformational changes are interpreted in terms of function but the language is repeatedly suggestive. Can they put their findings into a more quantitative form with statistical analysis? "The EDA thus suggests that the dynamics of CI and CIII2 are allosterically coupled."
(6) The authors write "We find that an increase in the lipid tail length decreases the relative stability of the SC (Figure S5C)" This is a critical point but I could not interpret Figure S5C consistently with this sentence. Can the authors explain this?
(7) The authors use a 6x6 and 15x15 lattice to analyze SC formation. The SC assembly has 6 units of E_strain favoring its assembly, which they take up to 4 kT. At 3 kT, the SC should be favored by 18 kT, or a Boltzmann factor of 10^8. With only 225 sites, specific and non-specific complex formation should be robust. Can the authors please check their numbers or provide a qualitative guide to the data that would make clear what I'm missing?
In summary, the qualitative data presented are interesting (especially the combination of molecular modeling with simpler Monte Carlo modeling aiding broader interpretation of the results) ... but confusing in terms of the non-standard presentation of membrane mechanics and the difficulty of this reviewer to interpret some of the underlying figures: especially, the thickness of the leaflets around the protein and the relative thickness of cardiolipin. Resolving the quantitative interpretation of the bilayer deformation would greatly enhance the significance of their Monte Carlo model of SC formation.
-

Reviewer #2 (Public review):
Summary:
The authors have used large-scale atomistic and coarse-grained molecular dynamics simulations on the respiratory chain complex and investigated the effect of the complex on the inner mitochondrial membrane. They have also used a simple phenomenological model to establish that the super complex (SC) assembly and stabilisation are driven by the interplay between the "entropic" forces due to strain energy and the enthalpies forces (specific and non-specific) between lipid and protein domains. The authors also show that the SC in the membrane leads to thinning and there is preferential localisation of certain lipids (Cardiolipin) in the annular region of the complex. The data reports that the SC assembly has an effect on the conformational dynamics of individual proteins making up the assembled complex …
Reviewer #2 (Public review):
Summary:
The authors have used large-scale atomistic and coarse-grained molecular dynamics simulations on the respiratory chain complex and investigated the effect of the complex on the inner mitochondrial membrane. They have also used a simple phenomenological model to establish that the super complex (SC) assembly and stabilisation are driven by the interplay between the "entropic" forces due to strain energy and the enthalpies forces (specific and non-specific) between lipid and protein domains. The authors also show that the SC in the membrane leads to thinning and there is preferential localisation of certain lipids (Cardiolipin) in the annular region of the complex. The data reports that the SC assembly has an effect on the conformational dynamics of individual proteins making up the assembled complex and they undergo "allosteric crosstalk" to maintain the stable functional complex. From their conformational analyses of the proteins (individual and while in the complex) and membrane "structural" properties (such as thinning/lateral organization etc) as well from the out of their phenomenological lattice model, the authors have provided possible implications and molecular origin about the function of the complex in terms of aspects such as charge currents in internal mitochondrion membrane, proton transport activity and ATP synthesis.
Strengths:
The work is bold in terms of undertaking modelling and simulation of such a large complex that requires simulations of about a million atoms for long time scales. This requires technical acumen and resources. Also, the effort to make connections to experimental readouts has to be appreciated (though it is difficult to connect functional pathways with limited (additive forcefield) simulations.
Weakness:
There are several weaknesses in the paper (please see the list below). Claims such as "entropic effect", "membrane strain energy" and "allosteric cross talks" are not properly supported by evidence and seem far-fetched at times. There are other weaknesses as well. Please see the list below.
(i) Membrane "strain energy" has been loosely used and no effort is made to explain what the authors mean by the term and how they would quantify it. If the membrane is simulated in stress-free conditions, where are strains building up from?
(ii) In result #1 (Protein membrane interaction modulates the lipid dynamics ....), I strongly feel that the readouts from simulations are overinterpreted. Membrane lateral organization in terms of lipids having preferential localisation is not new (see doi: 10.1021/acscentsci.8b00143) nor membrane thinning and implications to function (https://doi.org/10.1091/mbc.E20-12-0794). The distortions that are visible could be due to a mismatch in the number of lipids that need to be there between the upper and lower leaflets after the protein complex is incorporated. Also, the physiological membrane will have several chemically different lipids that will minimise such distortions as well as would be asymmetric across the leaflets - none of which has been considered. Connecting chain length to strain energy is also not well supported - are the authors trying to correlate membrane order (Lo vs Ld) with strain energy?
(iii) Entropic effect: What is the evidence towards the entropic effect? If strain energy is entropic, the authors first need to establish that. They discuss enthalpy-entropy compensation but there is no clear data or evidence to support that argument. The lipids will rearrange themselves or have a preference to be close to certain regions of the protein and that generally arises because of enthalpies reasons (see the body of work done by Carol Robinson with Mass Spec where certain lipids prefer proteins in the GAS phase, certainly there is no entropy at play there). I find the claims of entropic effects very unconvincing.
(iv) The changes in conformations dynamics are subtle as reported by the authors and the allosteric arguments are made based on normal mode analyses. In the complex, there are large overlapping regions between the CI, CIII2, and SCI/III2. I am not sure how the allosteric crosstalk claim is established in this work - some more analyses and data would be useful. Normal mode analyses (EDA) suggest that the motions are coupled and correlated - I am not convinced that it suggests that there is allosteric cross-talk.
(v) The lattice model should be described better and the rationale for choosing the equation needs to be established. Specific interactions look unfavourable in the equation as compared to non-specific interactions.
-

Reviewer #3 (Public review):
Summary:
In this contribution, the authors report atomistic, coarse-grained, and lattice simulations to analyze the mechanism of supercomplex (SC) formation in mitochondria. The results highlight the importance of membrane deformation as one of the major driving forces for SC formation, which is not entirely surprising given prior work on membrane protein assembly, but certainly of major mechanistic significance for the specific systems of interest.
Strengths:
The combination of complementary approaches, including an interesting (re)analysis of cryo-EM data, is particularly powerful and might be applicable to the analysis of related systems. The calculations also revealed that SC formation has interesting impacts on the structural and dynamical (motional correlation) properties of the individual protein …
Reviewer #3 (Public review):
Summary:
In this contribution, the authors report atomistic, coarse-grained, and lattice simulations to analyze the mechanism of supercomplex (SC) formation in mitochondria. The results highlight the importance of membrane deformation as one of the major driving forces for SC formation, which is not entirely surprising given prior work on membrane protein assembly, but certainly of major mechanistic significance for the specific systems of interest.
Strengths:
The combination of complementary approaches, including an interesting (re)analysis of cryo-EM data, is particularly powerful and might be applicable to the analysis of related systems. The calculations also revealed that SC formation has interesting impacts on the structural and dynamical (motional correlation) properties of the individual protein components, suggesting further functional relevance of SC formation. Overall, the study is rather thorough and highly creative, and the impact on the field is expected to be significant.
Weaknesses:
In general, I don't think the work contains any obvious weaknesses, although I was left with some questions.
-

-