Disentangling acute motor deficits and adaptive responses evoked by the loss of cerebellar output
Curation statements for this article:-
Curated by eLife
eLife Assessment
Using a unique cerebellar disruption approach in non-human primates, this study provides valuable new insight into how cerebellar inputs to the motor cortex contribute to reaching. The findings convincingly demonstrate that reaching movements following cerebellar disruption slow down because of both an acute deficit in producing muscle activity as well as a progressive decline in compensating for limb dynamics. This work will be of interest to neuroscientists and clinicians interested in cerebellar function and pathology.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Patients with cerebellar damage experience various motor impairments, but the specific sequence of primary and compensatory processes that contribute to these deficits remains unclear. To clarify this, we reversibly blocked cerebellar outflow in monkeys engaged in planar reaching tasks. This intervention led to a spatially selective reduction in hand velocity, primarily due to decreased muscle torque, especially in movements requiring high inter-joint torque coupling. When examining repeated reaches to the same target, we found that the reduced velocity resulted from both an immediate deficit and a gradually developing compensatory slowing to reduce passive inter-joint interactions. However, the slowed hand velocity did not account for the fragmented and variable movement trajectories observed during the cerebellar block. Our findings indicate that cerebellar impairment results in motor deficits due to both inadequate muscle torque and an altered motor control strategy for managing impaired limb dynamics. Additionally, impaired motor control elevates noise, which cannot be entirely mitigated through compensatory strategies.
Article activity feed
-

-

-

-

eLife Assessment
Using a unique cerebellar disruption approach in non-human primates, this study provides valuable new insight into how cerebellar inputs to the motor cortex contribute to reaching. The findings convincingly demonstrate that reaching movements following cerebellar disruption slow down because of both an acute deficit in producing muscle activity as well as a progressive decline in compensating for limb dynamics. This work will be of interest to neuroscientists and clinicians interested in cerebellar function and pathology.
-

Reviewer #1 (Public review):
Summary:
In a previous work Prut and colleagues had shown that during reaching, high frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report they extend their previous work by addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joint. More interestingly, the experiment revealed evidence for decomposition of the reaching movement, as well as an increase in the variance of the …
Reviewer #1 (Public review):
Summary:
In a previous work Prut and colleagues had shown that during reaching, high frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report they extend their previous work by addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joint. More interestingly, the experiment revealed evidence for decomposition of the reaching movement, as well as an increase in the variance of the trajectory.
Strengths:
This is a rare experiment in a non-human primate that assessed the importance of cerebellar input to the motor cortex during reaching.
Weaknesses:
None
-

Reviewer #2 (Public review):
This manuscript asks an interesting and important question: what part of 'cerebellar' motor dysfunction is an acute control problem vs a compensatory strategy to the acute control issue? The authors use a cerebellar 'blockade' protocol, consisting of high frequency stimuli applied to the cerebellar peduncle which is thought to interfere with outflow signals. This protocol was applied in monkeys performing center out reaching movements and has been published from this laboratory in several preceding studies. I found the take-home-message broadly convincing and clarifying - that cerebellar block reduces muscle activation acutely particularly in movements that involve multiple joints and therefore invoke interaction torques, and that movements progressively slow down to in effect 'compensate' for these acute …
Reviewer #2 (Public review):
This manuscript asks an interesting and important question: what part of 'cerebellar' motor dysfunction is an acute control problem vs a compensatory strategy to the acute control issue? The authors use a cerebellar 'blockade' protocol, consisting of high frequency stimuli applied to the cerebellar peduncle which is thought to interfere with outflow signals. This protocol was applied in monkeys performing center out reaching movements and has been published from this laboratory in several preceding studies. I found the take-home-message broadly convincing and clarifying - that cerebellar block reduces muscle activation acutely particularly in movements that involve multiple joints and therefore invoke interaction torques, and that movements progressively slow down to in effect 'compensate' for these acute tone deficits. The manuscript was generally well written, data were clear, convincing and novel. The key strengths are differentiating acute from sub-acute (within session but not immediate) kinematic consequences of cerebellar block.
-

Reviewer #3 (Public review):
Summary:
In their revised manuscript, Sinha and colleagues aim to identify distinct causes of motor impairments seen when perturbing cerebellar circuits. This goal is an important one, given the diversity of movement related phenotypes in patients with cerebellar lesion or injury, which are especially difficult to dissect given the chronic nature of the circuit damage. To address this goal, the authors use high-frequency stimulation (HFS) of the superior cerebellar peduncle in monkeys performing reaching movements. HFS provides an attractive approach for transiently disrupting cerebellar function previously published by this group. First, they find a reduction in hand velocities during reaching, which was more pronounced for outward versus inward movements. By modeling inverse dynamics, they find evidence …
Reviewer #3 (Public review):
Summary:
In their revised manuscript, Sinha and colleagues aim to identify distinct causes of motor impairments seen when perturbing cerebellar circuits. This goal is an important one, given the diversity of movement related phenotypes in patients with cerebellar lesion or injury, which are especially difficult to dissect given the chronic nature of the circuit damage. To address this goal, the authors use high-frequency stimulation (HFS) of the superior cerebellar peduncle in monkeys performing reaching movements. HFS provides an attractive approach for transiently disrupting cerebellar function previously published by this group. First, they find a reduction in hand velocities during reaching, which was more pronounced for outward versus inward movements. By modeling inverse dynamics, they find evidence that shoulder muscle torques are especially affected. Next, the authors examine the temporal evolution of movement phenotypes over successive blocks of HFS trials. Using this analysis, they find that in addition to the acute, specific effects on torques in early HFS trials, there was an additional progressive reduction in velocity during later trials, which they interpret as an adaptive response to the inability to effectively compensate for interaction torques during cerebellar block. Finally, the authors examine movement decomposition and trajectory, finding that even when low velocity reaches are matched to controls, HFS produces abnormally decomposed movements and higher than expected variability in trajectory.
Strengths:
Overall, this work provides important insight into how perturbation of cerebellar circuits can elicit diverse effects on movement across multiple timescales.
The HFS approach provides temporal resolution and enables analysis that would be hard to perform in the context of chronic lesions or slow pharmacological interventions. Thus, this study describes an important advance over prior methods of circuit disruption in the monkey, and their approach can be used as a framework for future studies that delve deeper into how additional aspects of sensorimotor control are disrupted (e.g., response to limb perturbations).
In addition, the authors use well-designed behavioral approaches and analysis methods to distinguish immediate from longer-term adaptive effects of HFS on behavior. Moreover, inverse dynamics modeling provides important insight into how movements with different kinematics and muscle dynamics might be differentially disrupted by cerebellar perturbation.
Remaining comments:
The argument that there are acute and adaptive effects to perturbing cerebellar circuits is compelling, but there seems to be a lost opportunity to leverage the fast and reversible nature of the perturbations to further test this idea and strengthen the interpretation. Specifically, the authors could have bolstered this argument by looking at the effects of terminating HFS - one might hypothesize that the acute impacts on joint torques would quickly return to baseline in the absence of HFS, whereas the longer-term adaptive component would persist in the form of aftereffects during the 'washout' period. As is, the reversible nature of the perturbation seems underutilized in testing the authors' ideas. While this experimental design was not implemented here, it seems like a good opportunity for future work using these approaches.
The analysis showing that there is a gradual reduction in velocity during what the authors call an adaptive phase is convincing. While it is still not entirely clear why disruption of movement during the adaptive phase is not seen for inward targets, despite the fact that many of the inward movements also exhibit large interaction torques, the authors do raise potential explanations in the Discussion.
-

Author response:
The following is the authors’ response to the previous reviews
Reviewer #1 (Public review):
Summary:
In a previous work Prut and colleagues had shown that during reaching, high frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report they extend their previous work by addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joint. More interestingly, the experiment revealed evidence for …
Author response:
The following is the authors’ response to the previous reviews
Reviewer #1 (Public review):
Summary:
In a previous work Prut and colleagues had shown that during reaching, high frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report they extend their previous work by addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joint. More interestingly, the experiment revealed evidence for decomposition of the reaching movement, as well as an increase in the variance of the trajectory.
Strengths:
This is a rare experiment in a non-human primate that assessed the importance of cerebellar input to the motor cortex during reaching.
Weaknesses:
None
Reviewer #1 (Recommendations for the authors):
The authors have answered my questions adequately and I have no further comments.
Reviewer #2 (Public review):
This manuscript asks an interesting and important question: what part of 'cerebellar' motor dysfunction is an acute control problem vs a compensatory strategy to the acute control issue? The authors use a cerebellar 'blockade' protocol, consisting of high frequency stimuli applied to the cerebellar peduncle which is thought to interfere with outflow signals. This protocol was applied in monkeys performing center out reaching movements and has been published from this laboratory in several preceding studies. I found the takehome-message broadly convincing and clarifying - that cerebellar block reduces muscle activation acutely particularly in movements that involve multiple joints and therefore invoke interaction torques, and that movements progressively slow down to in effect 'compensate' for these acute tone deficits. The manuscript was generally well written, data were clear, convincing and novel. The key strengths are differentiating acute from subacute (within session but not immediate) kinematic consequences of cerebellar block.
Reviewer #2 (Recommendations for the authors):
I think the manuscript is good as is. That said, it would have been nice to see more of the behavioral outcomes in Figure 5 (e.g. decomposition and trajectory variability) analyzed longitudinally like the velocity measurements in Fig. 4. This would clearly strengthen the insight into acute and compensatory components of cerebellar motor deficits.
The two behavioral measures of motor noise used in our study are movement decomposition and trajectory variability (Figure 5). Since trajectory variability is measured across trials we could not analyze this measure longitudinally as a function of trial number. However, following the reviewer’s advice, we examined movement
decomposition for successive trials in control vs. cerebellar block for movements to targets 2-4 similar to the analysis of hand velocity in figure 4. We found no interaction effect between trial sequence x cerebellar block on movement decomposition. This result is consistent with our conclusion that noisy joint activation occurs independently of adaptive slowing of multi-joint movements. We have updated our main text (lines 293-299) and supplementary information (supplementary figure S5 and supplementary table S8) to include this result.
Reviewer #3 (Public review):
Summary:
In their revised manuscript, Sinha and colleagues aim to identify distinct causes of motor impairments seen when perturbing cerebellar circuits. This goal is an important one, given the diversity of movement related phenotypes in patients with cerebellar lesion or injury, which are especially difficult to dissect given the chronic nature of the circuit damage. To address this goal, the authors use high-frequency stimulation (HFS) of the superior cerebellar peduncle in monkeys performing reaching movements. HFS provides an attractive approach for transiently disrupting cerebellar function previously published by this group. First, they find a reduction in hand velocities during reaching, which was more pronounced for outward versus inward movements. By modeling inverse dynamics, they find evidence that shoulder muscle torques are especially affected. Next, the authors examine the temporal evolution of movement phenotypes over successive blocks of HFS trials. Using this analysis, they find that in addition to the acute, specific effects on torques in early HFS trials, there was an additional progressive reduction in velocity during later trials, which they interpret as an adaptive response to the inability to effectively compensate for interaction torques during cerebellar block. Finally, the authors examine movement decomposition and trajectory, finding that even when low velocity reaches are matched to controls, HFS produces abnormally decomposed movements and higher than expected variability in trajectory.
Strengths:
Overall, this work provides important insight into how perturbation of cerebellar circuits can elicit diverse effects on movement across multiple timescales.
The HFS approach provides temporal resolution and enables analysis that would be hard to perform in the context of chronic lesions or slow pharmacological interventions. Thus, this study describes an important advance over prior methods of circuit disruption in the monkey, and their approach can be used as a framework for future studies that delve deeper into how additional aspects of sensorimotor control are disrupted (e.g., response to limb perturbations).
In addition, the authors use well-designed behavioral approaches and analysis methods to distinguish immediate from longer-term adaptive effects of HFS on behavior. Moreover, inverse dynamics modeling provides important insight into how movements with different kinematics and muscle dynamics might be differentially disrupted by cerebellar perturbation.
In this revised version of the manuscript, the authors have provided additional analyses and clarification that address several of the comments from the original submission.
Remaining comments:
The argument that there are acute and adaptive effects to perturbing cerebellar circuits is compelling, but there seems to be a lost opportunity to leverage the fast and reversible nature of the perturbations to further test this idea and strengthen the interpretation. Specifically, the authors could have bolstered this argument by looking at the effects of terminating HFS - one might hypothesize that the acute impacts on joint torques would quickly return to baseline in the absence of HFS, whereas the longer-term adaptive component would persist in the form of aftereffects during the 'washout' period. As is, the reversible nature of the perturbation seems underutilized in testing the authors' ideas. While this experimental design was not implemented here, it seems like a good opportunity for future work using these approaches.
We agree with the reviewer that examining the effect of the cerebellar block on immediate post-block washout trials in future studies will be insightful.
The analysis showing that there is a gradual reduction in velocity during what the authors call an adaptive phase is convincing. While it is still not entirely clear why disruption of movement during the adaptive phase is not seen for inward targets, despite the fact that many of the inward movements also exhibit large interaction torques, the authors do raise potential explanations in the Discussion.
The text in the Introduction and in the prior work developing the HFS approach overstates the selectivity of the perturbations. First, there is an emphasis on signals transmitted to the neocortex. As the authors state several times in the Discussion, there are many subcortical targets of the cerebellar nuclei as well, and thus it is difficult to disentangle target-specific behavioral effects using this approach. Second, the superior cerebellar peduncle contains both cerebellar outputs and inputs (e.g., spinocerebellar). Therefore, the selectivity in perturbing cerebellar output feels overstated. Readers would benefit from a more agnostic claim that HFS affects cerebellar communication with the rest of the nervous system, which would not affect the major findings of the study. In the revised manuscript, the authors do provide additional anatomical and evolutionary context and discuss potential limitations in the selectivity of HFS in the Materials and Methods. However, I feel that at least a brief mention of these caveats in the Introduction, where it is stated, "we then reversibly blocked cerebellar output to the motor cortex", would benefit the reader.
Following the advice of the reviewer, we have now revised the introduction section of our manuscript in the following way (lines 61-67):
“…We then reversibly disrupted cerebellar communication with other neural structures using high-frequency stimulation (HFS) of the superior cerebellar peduncle, assessing the impact of this perturbation on subsequent movements. Although our approach primarily affects cerebellar output to the motor cortex, it also disrupts fibers carrying input signals (e.g., spinocerebellar) and pathways to various subcortical targets (e.g., cerebellorubrospinal). Thus, our manipulation broadly interferes with cerebellar communication…”
Reviewer #3 (Recommendations for the authors):
Typo on line 102; "subs-sessions"
We have corrected this typographical error in our revised manuscript (line 106).
-

-

eLife Assessment
Using a unique cerebellar disruption approach in non-human primates, this study provides valuable new insight into how cerebellar inputs to the motor cortex contribute to reaching. The findings convincingly demonstrate that reaching movements following cerebellar disruption slow down because of both an acute deficit in producing muscle activity as well as a progressive decline in compensating for limb dynamics. This work will be of interest to neuroscientists and clinicians interested in cerebellar function and pathology.
-

Reviewer #1 (Public review):
Summary:
In a previous work Prut and colleagues had shown that during reaching, high frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report they extend their previous work by addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joint. More interestingly, the experiment revealed evidence for decomposition of the reaching movement, as well as an increase in the variance of the …
Reviewer #1 (Public review):
Summary:
In a previous work Prut and colleagues had shown that during reaching, high frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report they extend their previous work by addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joint. More interestingly, the experiment revealed evidence for decomposition of the reaching movement, as well as an increase in the variance of the trajectory.
Strengths:
This is a rare experiment in a non-human primate that assessed the importance of cerebellar input to the motor cortex during reaching.
Weaknesses:
None
-

Reviewer #2 (Public review):
This manuscript asks an interesting and important question: what part of 'cerebellar' motor dysfunction is an acute control problem vs a compensatory strategy to the acute control issue? The authors use a cerebellar 'blockade' protocol, consisting of high frequency stimuli applied to the cerebellar peduncle which is thought to interfere with outflow signals. This protocol was applied in monkeys performing center out reaching movements and has been published from this laboratory in several preceding studies. I found the take-home-message broadly convincing and clarifying - that cerebellar block reduces muscle activation acutely particularly in movements that involve multiple joints and therefore invoke interaction torques, and that movements progressively slow down to in effect 'compensate' for these acute …
Reviewer #2 (Public review):
This manuscript asks an interesting and important question: what part of 'cerebellar' motor dysfunction is an acute control problem vs a compensatory strategy to the acute control issue? The authors use a cerebellar 'blockade' protocol, consisting of high frequency stimuli applied to the cerebellar peduncle which is thought to interfere with outflow signals. This protocol was applied in monkeys performing center out reaching movements and has been published from this laboratory in several preceding studies. I found the take-home-message broadly convincing and clarifying - that cerebellar block reduces muscle activation acutely particularly in movements that involve multiple joints and therefore invoke interaction torques, and that movements progressively slow down to in effect 'compensate' for these acute tone deficits. The manuscript was generally well written, data were clear, convincing and novel. The key strengths are differentiating acute from sub-acute (within session but not immediate) kinematic consequences of cerebellar block.
-

Reviewer #3 (Public review):
Summary:
In their revised manuscript, Sinha and colleagues aim to identify distinct causes of motor impairments seen when perturbing cerebellar circuits. This goal is an important one, given the diversity of movement related phenotypes in patients with cerebellar lesion or injury, which are especially difficult to dissect given the chronic nature of the circuit damage. To address this goal, the authors use high-frequency stimulation (HFS) of the superior cerebellar peduncle in monkeys performing reaching movements. HFS provides an attractive approach for transiently disrupting cerebellar function previously published by this group. First, they find a reduction in hand velocities during reaching, which was more pronounced for outward versus inward movements. By modeling inverse dynamics, they find evidence …
Reviewer #3 (Public review):
Summary:
In their revised manuscript, Sinha and colleagues aim to identify distinct causes of motor impairments seen when perturbing cerebellar circuits. This goal is an important one, given the diversity of movement related phenotypes in patients with cerebellar lesion or injury, which are especially difficult to dissect given the chronic nature of the circuit damage. To address this goal, the authors use high-frequency stimulation (HFS) of the superior cerebellar peduncle in monkeys performing reaching movements. HFS provides an attractive approach for transiently disrupting cerebellar function previously published by this group. First, they find a reduction in hand velocities during reaching, which was more pronounced for outward versus inward movements. By modeling inverse dynamics, they find evidence that shoulder muscle torques are especially affected. Next, the authors examine the temporal evolution of movement phenotypes over successive blocks of HFS trials. Using this analysis, they find that in addition to the acute, specific effects on torques in early HFS trials, there was an additional progressive reduction in velocity during later trials, which they interpret as an adaptive response to the inability to effectively compensate for interaction torques during cerebellar block. Finally, the authors examine movement decomposition and trajectory, finding that even when low velocity reaches are matched to controls, HFS produces abnormally decomposed movements and higher than expected variability in trajectory.
Strengths:
Overall, this work provides important insight into how perturbation of cerebellar circuits can elicit diverse effects on movement across multiple timescales.
The HFS approach provides temporal resolution and enables analysis that would be hard to perform in the context of chronic lesions or slow pharmacological interventions. Thus, this study describes an important advance over prior methods of circuit disruption in the monkey, and their approach can be used as a framework for future studies that delve deeper into how additional aspects of sensorimotor control are disrupted (e.g., response to limb perturbations).
In addition, the authors use well-designed behavioral approaches and analysis methods to distinguish immediate from longer-term adaptive effects of HFS on behavior. Moreover, inverse dynamics modeling provides important insight into how movements with different kinematics and muscle dynamics might be differentially disrupted by cerebellar perturbation.
In this revised version of the manuscript, the authors have provided additional analyses and clarification that address several of the comments from the original submission.
Remaining comments:
The argument that there are acute and adaptive effects to perturbing cerebellar circuits is compelling, but there seems to be a lost opportunity to leverage the fast and reversible nature of the perturbations to further test this idea and strengthen the interpretation. Specifically, the authors could have bolstered this argument by looking at the effects of terminating HFS - one might hypothesize that the acute impacts on joint torques would quickly return to baseline in the absence of HFS, whereas the longer-term adaptive component would persist in the form of aftereffects during the 'washout' period. As is, the reversible nature of the perturbation seems underutilized in testing the authors' ideas. While this experimental design was not implemented here, it seems like a good opportunity for future work using these approaches.
The analysis showing that there is a gradual reduction in velocity during what the authors call an adaptive phase is convincing. While it is still not entirely clear why disruption of movement during the adaptive phase is not seen for inward targets, despite the fact that many of the inward movements also exhibit large interaction torques, the authors do raise potential explanations in the Discussion.
The text in the Introduction and in the prior work developing the HFS approach overstates the selectivity of the perturbations. First, there is an emphasis on signals transmitted to the neocortex. As the authors state several times in the Discussion, there are many subcortical targets of the cerebellar nuclei as well, and thus it is difficult to disentangle target-specific behavioral effects using this approach. Second, the superior cerebellar peduncle contains both cerebellar outputs and inputs (e.g., spinocerebellar). Therefore, the selectivity in perturbing cerebellar output feels overstated. Readers would benefit from a more agnostic claim that HFS affects cerebellar communication with the rest of the nervous system, which would not affect the major findings of the study. In the revised manuscript, the authors do provide additional anatomical and evolutionary context and discuss potential limitations in the selectivity of HFS in the Materials and Methods. However, I feel that at least a brief mention of these caveats in the Introduction, where it is stated, "we then reversibly blocked cerebellar output to the motor cortex", would benefit the reader.
-

Author response:
The following is the authors’ response to the original reviews
Summary of Revisions
We sincerely thank the editors and reviewers for their thorough assessment and constructive feedback, which has greatly improved our manuscript. We have carefully addressed all concerns as summarized below:
In response to the requests made by Reviewer #1:
• Clarified task design and acknowledged its limitations regarding endpoint accuracy control.
• Included analysis comparing the effects of cerebellar block on within-trial versus inter-trial movements.
• Clearly defined target groupings, replacing the term “single-joint” with “movements with low coupling torques” and “multi-joint” with “movements with high coupling torques”: definitions which are now supported by a supplementary material describing the net torque data as a function of …
Author response:
The following is the authors’ response to the original reviews
Summary of Revisions
We sincerely thank the editors and reviewers for their thorough assessment and constructive feedback, which has greatly improved our manuscript. We have carefully addressed all concerns as summarized below:
In response to the requests made by Reviewer #1:
• Clarified task design and acknowledged its limitations regarding endpoint accuracy control.
• Included analysis comparing the effects of cerebellar block on within-trial versus inter-trial movements.
• Clearly defined target groupings, replacing the term “single-joint” with “movements with low coupling torques” and “multi-joint” with “movements with high coupling torques”: definitions which are now supported by a supplementary material describing the net torque data as a function of the targets.
• Added detailed descriptions of trial success criteria, based on timing, and positional constraints.
• Expanded figures illustrating the effect of the cerebellar block on movement decomposition and variability in joint space and across different target directions.
In response to the requests made by Reviewer #2:
• Included an explicit discussion highlighting why the acute reduction in muscle torque during cerebellar block is likely due to agonist weakness rather than cocontraction, emphasizing the rationale behind our torque-centric analysis.
• Clearly defined trial success criteria and included the timing and accuracy constraints used in our study.
• Clarified our rationale for grouping targets based on shoulder flexion/extension, clearly justified by interaction torque analysis.
• Revised the caption and legend of Figure 3d for clarity and included partial correlation results to account for the variability across monkeys for the analysis of reduction in hand velocity vs. coupling torque in control.
In response to the requests made by Reviewer #3:
• Included electrophysiological validation of the accuracy of targeting the superior cerebellar peduncle from one of the monkeys used in the experiment.
• Provided new analyses comparing movement decomposition and variability between slower and faster movements within the cerebellar block condition.
• Revised manuscript text to clarify terminology and clearly explained the rationale behind target groupings and torque analyses.
• Expanded discussion sections to better explain the relationships between timing deficits, movement decomposition, trajectory variability, and faulty motor commands.
• Clarified methodological choices regarding our analysis timeframe and acknowledged limitations related to the distinction between feedforward and feedback control.
Reviewer #1 (Public review):
Summary:
In a previous work, Prut and colleagues had shown that during reaching, high-frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report, they extend their previous work by the addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joints. More interestingly, the experiment revealed evidence for the decomposition of the reaching movement, as well as an increase in the variance of the trajectory.
Strengths:
This is a rare experiment in a non-human primate that assessed the importance of cerebellar input to the motor cortex during reaching.
We thank the reviewer for their positive feedback on our study. We particularly appreciate their recognition of the novelty and importance of our experimental approach in non-human primates, as well as their insightful summary of our key findings.
Weaknesses:
My major concerns are described below.
If I understand the task design correctly, the monkeys did not need to stop their hand at the target. I think this design may be suboptimal for investigating the role of the cerebellum in control of reaching because a number of earlier works have found that the cerebellum's contributions are particularly significant as the movement ends, i.e., stopping at the target. For example, in mice, interposed nucleus neurons tend to be most active near the end of the reach that requires extension, and their activation produces flexion forces during the reach (Becker and Person 2019). Indeed, the inactivation of interposed neurons that project to the thalamus results in overshooting of reaching movements (Low et al. 2018). Recent work has also found that many Purkinje cells show a burst-pause pattern as the reach nears its endpoint, and stimulation of the mossy fibers tends to disrupt endpoint control (Calame et al. 2023). Thus, the fact that the current paper has no data regarding endpoint control of the reach is puzzling to me.
We appreciate the reviewer’s point that cerebellar contributions can be particularly critical near the endpoint of a reach. In our task design, monkeys were indeed required to hold at the target briefly—100 ms for Monkeys S and P, and 150 ms for Monkeys C and M—before receiving the reward. However, given the size of the targets and the velocity of movements, it often happened that the monkeys didn’t have to stop their movements fully to obtain the reward. Importantly, we relaxed the task’s requirements (by increasing the target size and reducing the temporal constraints) to enable the monkeys to perform a sufficient number of successful trials under both the control and the cerebellar block conditions. This was necessary as we found that strict criteria regarding these parameters yielded a very low success rate in the cerebellar block condition. Nevertheless, as we appreciate now, this task design is suboptimal for studying endpoint accuracy which is an important aspect of cerebellar control. In the methods section of our revised manuscript, we have clarified this aspect of the task design and acknowledged that it is sub-optimal for examining the role of the cerebellum in end-point control (lines 475-485). The task design of our future studies will explicitly address this point more carefully.
Because stimulation continued after the cursor had crossed the target, it is interesting to ask whether this disruption had any effects on the movements that were task-irrelevant. The reason for asking this is because we have found that whereas during task-relevant eye or tongue movements the Purkinje cells are strongly modulated, the modulations are much more muted when similar movements are performed but are task-irrelevant (Pi et al., PNAS 2024; Hage et al. Biorxiv 2024). Thus, it is interesting to ask whether the effects of stimulation were global and affected all movements, or were the effects primarily concerned with the task-relevant movements.
This is an insightful suggestion. The behavioral task in the present study was designed with a focus on task-relevant, reward-associated reaching movements. Nevertheless, we also have data on the inter-trial movements (e.g., return-to-center reaches) under continued cerebellar stimulation, which were not directly associated with reward. In response to the reviewer’s comment, we compared the effects of cerebellar block on endpoint velocities between these two types of movements. We found that reductions in peak hand velocity during inter-trial movements were significantly smaller than those observed during the target directed reaches. We have updated the Results section of our manuscript (lines 125-137) and expanded our supplementary document (Supplementary Figure S1) to include this analysis.
If the schematic in Figure 1 is accurate, it is difficult for me to see how any of the reaching movements can be termed single joint. In the paper, T1 is labeled as a single joint, and T2T4 are labeled as dual-joint. The authors should provide data to justify this.
The reviewer is correct. Movements to all targets involved both shoulder and elbow joints, but the degree to which each joint participated varied in a targetspecific manner. In our original manuscript, we used the term “single-joint” to refer to movements in which one joint was nearly stationary, resulting in minimal coupling torque at the adjacent joint. Specifically, for Targets 1 and 5, the net torque—and thus acceleration— at the elbow was negligible, causing the shoulder to experience low coupling torques (as illustrated in Figure 3c of our revised manuscript). Following this comment and to avoid confusion, we have now explained this explicitly in the revised manuscript (lines 178-187). This is supported by Supplementary Figure S2 demonstrating the net torques at the shoulder and elbow for movements to each target. We have also replaced the term ‘singlejoint movements’ and ‘multi-joint movements’ with ‘movements with low coupling torques’ and ‘movements with high coupling torques’ respectively in our revised manuscript (lines 178-180, 204-207, 225-227, 230-232, 305-307, and 362-365).
Because at least part of this work was previously analyzed and published, information should be provided regarding which data are new.
While some of the same animals and stimulation protocol were presented in prior work, the inverse-dynamics modeling, the analyses exploring progressive velocity changes across trials under a cerebellar block, and the relationship of motor noise to movement velocity are newly reported in this manuscript. We have included a clear statement in the Methods section specifying which components of the dataset and analyses are entirely new (lines 582-589).
Reviewer #1 (Recommendations for the authors):
(1) Before the results are presented, it is useful to present the experimental paradigm in more detail. For example, after the center-out movement was completed, was the monkey required to hold at the target location? How did the next trial begin (re-centering movement)? Next, specify the stimulation protocol, noting that each session was divided into 3-4 blocks of stimulation and not stimulation, with each block 50-80 trials.
We have updated the results section of our revised manuscript (lines 91-104) to present the experimental paradigm in more detail according to the reviewer’s advice.
(2) Figure 1. Hand velocity does not show how the reach was completed. Did the subjects stop at the target or simply shoot through it and turn around without stopping? Why are the traces cut off?
Monkeys were indeed required to hold at the target briefly (100-150 ms) before receiving the reward. However, given the size of the targets and the velocity of movements, it often happened that the monkeys didn’t have to stop their movements fully to obtain the reward. The hand velocity profile shown in Figure 1b and the torque profiles shown in Figures 2a and 2b correspond to the period from movement onset to the entry of the control cursor into the peripheral target which marked the end of the movement for the trial. Since the monkeys didn’t have to stop their movements fully for the trial to end, the traces appear cut off at the beginning of the deceleration/stopping phase of the movement. We have updated the captions of Figures 1b, 2a, and 2b to include this information (lines 869-872 and 882-884).
(3) Maybe state that the data regarding reaction times are not presented because of the task design in which the go signal was predictable.
In monkeys M and C, the timing of the go signal was fixed and therefore predictable. Furthermore, they were also allowed a grace period of 200 ms before the go signal to facilitate predictive timing which often resulted in negative reaction times. However, in Monkeys S and P, the go signal was variable in timing and the monkeys were not allowed to initiate the movements before the go signal. In our previous studies (Nashef et al., 2019; Israely et al. 2025), we reported increased reaction times under cerebellar block. However, since the present study focuses specifically on execution-related motor deficits, we did not analyze reaction time data.
(4) Please provide the data and analysis regarding the entire reach, including the period after the cursor crosses the target and returns to the center position.
We compared the peak hand velocity of the target-directed movements to the inter-trial return-to-center movements. Cerebellar block produced significantly smaller reductions in peak hand velocity during inter-trial movements compared to within-trial reaches. The results section of our revised manuscript (lines 125137) and the supplementary material (Supplementary Figure S1) have been updated accordingly. While the behavioral task in the present study was designed with a focus on task-relevant, reward-associated reaching movements, it will be interesting to examine in detail the effect of cerebellar block on spontaneous movements in a future study.
(5) Figure 5. To illustrate the decomposition of multijoint movements into a sequence of single joint movements, I suggest plotting movements in joint space (in addition to Cartesian space as you have done now). The results in Figure 5 are most interesting and thus should be expanded. Please provide this data using the format in Figure 1C, that is, as a function of direction.
Following the reviewer’s suggestion, we have plotted sample trajectories in joint-velocity (Supplementary Figures 3a and b) and position space (Supplementary Figures 4a and b) to highlight the decomposition of multi-joint movements and increased inter-trial trajectory variability respectively during the cerebellar block. Additionally, we also analyzed movement decomposition and trajectory variability as a function of target direction (Supplementary Figures 3c and 4c respectively). The corresponding text in the Results section has been updated accordingly (lines 256-261, 267-271, 277-278 and 280-288).
Reviewer #2 (Public review):
This manuscript asks an interesting and important question: what part of 'cerebellar' motor dysfunction is an acute control problem vs a compensatory strategy to the acute control issue? The authors use a cerebellar 'blockade' protocol, consisting of high-frequency stimuli applied to the cerebellar peduncle which is thought to interfere with outflow signals. This protocol was applied in monkeys performing center outreaching movements and has been published from this laboratory in several preceding studies. I found the takehome-message broadly convincing and clarifying - that cerebellar block reduces muscle activation acutely particularly in movements that involve multiple joints and therefore invoke interaction torques, and that movements progressively slow down to in effect 'compensate' for these acute tone deficits. The manuscript was generally well written, and the data was clear, convincing, and novel. My comments below highlight suggestions to improve clarity and sharpen some arguments.
We thank the reviewer for their thoughtful and constructive feedback. We are grateful for their recognition of the significance of our findings regarding acute and compensatory motor responses following a cerebellar block.
Primary comments:
(1) Torque vs. tone: Is it known whether this type of cerebellar blockade is reducing muscle tone or inducing any type of acute co-contraction that could influence limb velocity through mechanisms different than 'atonia'? If so, the authors should discuss this information in the discussion section starting around line 336, and clarify that this motivates (if it does) the focus on 'torques' rather than muscle activation. Relatedly, besides the fact that there are joints involved, is there a reason there is so much emphasis on torque per se? If the muscle is deprived of sufficient drive, it would seem that it would be more straightforward to conceptualize the deficit as one of insufficient timed drive to a set of muscles than joint force. Some text better contextualizing the choices made here would be sufficient to address this concern. I found statements like those in the introduction "hand velocity was low initially, reflecting a primary muscle torque deficit" to be lacking in substance. Either that statement is self-evident or the alternative was not made clear. Finally, emphasize that it is a loss of self-generated torque at the shoulder that accounts for the velocity deficits. At times the phrasing makes it seem that there is a loss of some kind of passive torque.
We appreciate the reviewer's emphasis on distinguishing between reduced muscle tone and altered co-contraction patterns as potential explanations for decreased limb velocity. Our focus on torques per se arises from previous studies suggesting that a core deficit in cerebellar ataxia is impaired prediction of passive coupling torques (Bastian et al., 1996). In our study, we demonstrate that motor deficits in cerebellar ataxia result in fact from both the inability to compensate for passive coupling torques and an acute insufficiency in the ability to generate active muscle torques.
The muscle torque, representing the sum of all muscle forces acting at a joint, can indeed be reduced by any of the two mechanisms: (i) co-contraction of agonist and antagonist muscles, and/or (ii) insufficient agonist muscle activity (i.e., agonist weakness). In cerebellar ataxia, co-contraction has been proposed as a simplifying strategy to stabilize stationary joints during decomposed multi-joint movements (Bastian et al., 1996). In our experiments, this strategy would likely emerge gradually following cerebellar block similar to the adaptive slowing of movements aimed at reducing inter-joint interactions. However, we found that irrespective of the magnitude of coupling torques involved, reduction in the velocity of movements also occurred immediately following cerebellar block—a pattern less consistent with gradually emerging compensatory strategies. We therefore argue that this acute onset of movement slowing was mainly driven by agonist weakness. Our argument is further supported by previous studies which attributed reduced agonist muscle activity as a cause for the slowing of voluntary movements in individuals with cerebellar lesions (Hallet et al. 1991; Wild et al., 1996). Additionally, early studies have also reported muscle weakness (asthenia) and hypotonia acutely following cerebellar injury in humans (Haines et al., 2007) and experimental lesions in animals (Luciani, 1893; Bremer et al., 1935; Fulton & Dow, 1937; Granit et al., 1955).
We have modified the discussion section of our revised manuscript (lines 366-376) to explain/clarify this. Additionally, we have also underscored that the observed velocity deficits primarily reflect a reduction of self-generated torque at the shoulder (whether acute or adaptive), rather than any reduction in passive torque (lines 350-352).
(2) Please clarify some of the experimental metrics: Ln 94 RESULTS. The success rate is used as a primary behavioral readout, but what constitutes success is not clearly defined in the methods. In addition to providing a clear definition in the methods section, it would also be helpful for the authors to provide a brief list of criteria used to determine a 'successful' movement in the results section before the behavioral consequences of stimulation are described. In particular, the time and positional error requirements should be clear.
Successful trials were defined as trials in which monkeys didn’t leave the center position before the “Go” signal and entered the peripheral target within a permitted movement time. We have updated the results (lines 91-104) and methods (lines 475-485) section of our revised manuscript to include (i) the timing criteria of each phase of the trials and (ii) the size of the peripheral targets indicating the tolerance for endpoint accuracy.
(3) Based on the polar plot in Figure 1c, it seemed odd to consider Targets 1-4 outward and 5-8 inward movements, when 1 and 5 are side-to-side. Is there a rationale for this grouping or might results be cleaner by cleanly segregating outward (targets 2-4) and inward (targets 6-8) movements? Indeed, by Figure 3 where interaction torques are measured, this grouping would seem to align with the hypothesis much more cleanly since it is with T2,T3,and T4 where clear coupling torques deficits are seen with cerebellar block.
We acknowledge the reviewer's observation regarding the classification of targets 1 and 5 as side-to-side movements rather than strictly "outward" or "inward." In the initial section of our results, we grouped the targets based on shoulder joint movements: "outward" targets involved shoulder flexion, while "inward" targets involved shoulder extension. This classification highlighted the more pronounced effect of cerebellar block on movements requiring shoulder flexion compared to those requiring shoulder extension. For subsequent analyses, we focused on the effects of cerebellar block on movements to "outward" targets, which included directions involving low (target 1) or high (targets 2–4) coupling torques. To clarify this aspect, we have revised our manuscript to explain our definition of "outward" (targets 1–4) and "inward" (targets 5–8) target groupings based on shoulder flexion and extension movements respectively (lines 117-120).
(4) I did not follow Figure 3d. Both the figure axis labels and the description in the main text were difficult to follow. Furthermore, the color code per animal made me question whether the linear regression across the entire dataset was valid, or would be better performed within animal, and the regressions summarized across animals. The authors should look again at this section and figure.
We have revised the legend of Figure 3d to include a detailed explanation of how the value along each axis is computed (lines 908-920 of the revised manuscript). Please note that the color coding of the data points is as per the target number (T1-T4) and not the monkey number (as denoted in the figure legend). Also, pooling of data across monkeys was done after confirming that data from each animal expressed a similar trend. Specifically, the correlation coefficients were all positive but statistically significant in 3 out of the 4 monkeys. Following the reviewers’ feedback, we now performed a partial correlation analysis (which controls for the variability across monkeys) and found a significant correlation (r = 0.32, p < 0.001) between reduction in peak hand velocities during cerebellar block and the net coupling torque impulse. We have updated the manuscript to include the result of the partial correlation analysis (lines 173-176).
(5) Line 206+ The rationale for examining movement decomposition with a cerebellar block is presented as testing the role of the cerebellum in timing. Yet it is not spelled out what movement decomposition and trajectory variability have to do with motor timing per se.
The reviewer is right and the relations between timing, decomposition and variability need to be explicitly explained. In the results section of our revised manuscript, we have explained how decomposed movements and trajectory variability may reflect impaired temporal coordination across multiple joints—a critical cerebellar function (lines 235-244).
Reviewer #2 (Recommendations for the authors):
(1) Rephrase the findings, starting Line 232. Here the authors state, "Next, we asked whether movement decomposition was mainly due to lower hand velocities. We therefore selected a subset of control trials that matched the cerebellar block trials in their peak velocity. However, even though movement decomposition in these control trials was higher compared to all control trials, it was still significantly lower than velocity matched cerebellar block trials." I suggest inverting the final sentence to: "Movement decomposition in control trials was significantly lower than velocity-matched cerebellar block trials, even though these control trials themselves had somewhat higher decomposition indices than all control trials together." A similar issue pops up with trajectory variability below that simply requires some editing to be less clunky.
Following the reviewer’s suggestion, we have revised the sentences related to movement decomposition and trajectory variability. These sentences now reads as follows:
(lines 267-271 in the revised manuscript): “Movement decomposition in control trials was significantly lower than velocity-matched cerebellar block trials (p < 0.001; Figure 5c), even though these control trials themselves had 11.0% (CI [5.2, 17.0], p = 0.03) higher decomposition than the mean value calculated across all control trials.”
(lines 280-288 in the revised manuscript): “ When we compared the subset of velocitymatched control and cerebellar block trials, we found that cerebellar block trials exhibited 34.6% (CI [26.2, 43.2], p < 0.001) higher trajectory variability (Figure 5e). Normally, slower movements are also less variable due to the speed-accuracy tradeoff (Plamondon and Alimi 1997). Indeed, the trajectory variability in this subset of slower control trials was 5.5% (CI [0.9, 9.9], p = 0.02) lower than that of all control trials. In other words, despite slower movements, cerebellar block led to increased trajectory variability.”
(2) Typo: Ln 73 sequences, not sequence.
Typo error was corrected (line 75 of revised manuscript).
Reviewer #3 (Public review):
Summary:
In their manuscript, "Disentangling acute motor deficits and adaptive responses evoked by the loss of cerebellar output," Sinha and colleagues aim to identify distinct causes of motor impairments seen when perturbing cerebellar circuits. This goal is an important one, given the diversity of movement-related phenotypes in patients with cerebellar lesions or injuries, which are especially difficult to dissect given the chronic nature of the circuit damage. To address this goal, the authors use high-frequency stimulation (HFS) of the superior cerebellar peduncle in monkeys performing reaching movements. HFS provides an attractive approach for transiently disrupting cerebellar function previously published by this group. First, they found a reduction in hand velocities during reaching, which was more pronounced for outward versus inward movements. By modeling inverse dynamics, they find evidence that shoulder muscle torques are especially affected. Next, the authors examine the temporal evolution of movement phenotypes over successive blocks of HFS trials. Using this analysis, they find that in addition to the acute, specific effects on muscle torques in early HFS trials, there was an additional progressive reduction in velocity during later trials, which they interpret as an adaptive response to the inability to effectively compensate for interaction torques during cerebellar block. Finally, the authors examine movement decomposition and trajectory, finding that even when low-velocity reaches are matched to controls, HFS produces abnormally decomposed movements and higher than expected variability in trajectory.
Strengths:
Overall, this work provides important insight into how perturbation of cerebellar circuits can elicit diverse effects on movement across multiple timescales.
The HFS approach provides temporal resolution and enables analysis that would be hard to perform in the context of chronic lesions or slow pharmacological interventions. Thus, this study describes an important advance over prior methods of circuit disruption, and their approach can be used as a framework for future studies that delve deeper into how additional aspects of sensorimotor control are disrupted (e.g., response to limb perturbations).
In addition, the authors use well-designed behavioral approaches and analysis methods to distinguish immediate from longer-term adaptive effects of HFS on behavior. Moreover, inverse dynamics modeling provides important insight into how movements with different kinematics and muscle dynamics might be differentially disrupted by cerebellar perturbation.
We thank the reviewer for their detailed assessment and thoughtful comments and greatly appreciate their positive feedback.
Weaknesses:
The argument that there are acute and adaptive effects to perturbing cerebellar circuits is compelling, but there seems to be a lost opportunity to leverage the fast and reversible nature of the perturbations to further test this idea and strengthen the interpretation. Specifically, the authors could have bolstered this argument by looking at the effects of terminating HFS - one might hypothesize that the acute impacts on muscle torques would quickly return to baseline in the absence of HFS, whereas the longer-term adaptive component would persist in the form of aftereffects during the 'washout' period. As is, the reversible nature of the perturbation seems underutilized in testing the authors' ideas.
We agree that our approach could more explicitly exploit the rapid reversibility of high-frequency stimulation (HFS) by examining post-stimulation ‘washout’ periods. However, for the present dataset, we ended the session after the set of cerebellar block trials without using an explicit washout period. We plan to study the effect of the cerebellar block on immediate post-block washout trials in the future.
The analysis showing that there is a gradual reduction in velocity during what the authors call an adaptive phase is convincing. That said, the argument is made that this is due to difficulty in compensating for interaction torques. Even if the inward targets (i.e., targets 68) do not show a deficit during the acute phase, these targets still have significant interaction torques (Figure 3c). Given the interpretation of the data as presented, it is not clear why disruption of movement during the adaptive phase would not be seen for these targets as well since they also have large interaction torques. Moreover, it is difficult to delve into this issue in more detail, as the analyses in Figures 4 and 5 omit the inward targets.
The reviewer is right and movements to Targets 6–8 (inward) were seemingly unaffected despite also involving significant interaction torques. Specifically, we noted that while outward targets (2–4) tend to involve higher coupling torque impulses on average, this alone does not fully explain the differential impact of cerebellar block, as illustrated by discrepancies at the individual target level (e.g., target 7 vs. target 1). We propose two possible explanations: (1) a bias toward shoulder flexion in the effect of cerebellar block—consistent with earlier studies showing ipsilateral flexor activation or tone changes following stimulation or lesioning of the deep cerebellar nuclei; and (2) posture-related facilitation of inward (shoulder extension) movements from the central starting position. This point is addressed in the Discussion section (lines 404-433 in the revised manuscript).
The text in the Introduction and in the prior work developing the HFS approach overstates the selectivity of the perturbations. First, there is an emphasis on signals transmitted to the neocortex. As the authors state several times in the Discussion, there are many subcortical targets of the cerebellar nuclei as well, and thus it is difficult to disentangle target-specific behavioral effects using this approach. Second, the superior cerebellar peduncle contains both cerebellar outputs and inputs (e.g., spinocerebellar). Therefore, the selectivity in perturbing cerebellar output feels overstated. Readers would benefit from a more agnostic claim that HFS affects cerebellar communication with the rest of the nervous system, which would not affect the major findings of the study.
The reviewer is right that the superior cerebellar peduncle carries both descending and ascending fibers, and that cerebellar nuclei project to subcortical as well as cortical targets. Therefore, we cannot rule out the fact that the effect of HFS may be mediated in part through pathways other than the cerebello-thalamo-cortical pathway (as mentioned in the Discussion section). However, it is also important to note that in primates the cerebellar-thalamo-cortical (CTC) pathway greatly expanded (at the expense of the cerbello-rubro-spinal tract) in mediating cerebellar control of voluntary movements (Horne and Butler, 1995). The cerebello-subcortical pathways diminished in importance over the course of evolution (Nathan and Smith, 1982, Padel et al., 1981, ten Donkelaar, 1988). Previously we found that the ascending spinocerebellar axons which enter the cerebellum through the superior cerebellar peduncle (SCP) are weakly task-related and the descending system is quite small (Cohen et al, 2017). We have clarified these points and acknowledged that HFS disrupts cerebellar communication broadly, rather than solely the cerebellothalamo-cortical pathway in the methods section of our revised manuscript (lines 531544).
The text implies that increased movement decomposition and variability must be due to noise. However, this assumption is not tested. It is possible that the impairments observed are caused by disrupted commands, independent of whether these command signals are noisy. In other words, commands could be low noise but still faulty.
We recognize the reviewer’s concern about linking movement decomposition and trial-to-trial trajectory variability with motor noise. We interpret these motor abnormalities as a form of motor noise in the sense that they are generated by faulty motor commands. We draw our interpretation from the findings of previous research work which show that the cerebellum aids in the state estimation of the limb and subsequent generation of accurate feedforward commands. Therefore, disruption of the cerebellar output may lead to faulty motor commands resulting in the observed asynchronous joint activations (i.e., movement decomposition) and unpredictable trajectories (i.e., increased trial-to-trial variability). Both observed deficits resemble increased motor noise. This point is presented in our Discussion section (lines 436-458 of the revised manuscript),
Throughout the text, the use of the term 'feedforward control' seems unnecessary. To dig into the feedforward component of the deficit, the authors could quantify the trajectory errors only at the earliest time points (e.g., in Figure 5d), but even with this analysis, it is difficult to disentangle feedforward- and feedback-mediated effects when deficits are seen throughout the reach. While outside the scope of this study, it would be interesting to explore how feedback responses to limb perturbation are affected in control versus HFS conditions. However, as is, these questions are not explored, and the claim of impaired feedforward control feels overstated.
We agree that to strictly focus on feedforward control, we could have examined the measured variables in the first 50-100 ms of the movement which has been shown to be unaffected by feedback responses (Pruszynski et al. 2008, Todorov and Jordan 2002, Pruszynski and Scott 2012, Crevecoeur et al. 2013). However, in our task, the amplitude of movements made by the monkeys was small, and therefore the response measures in the first 50-100 ms were too small for a robust estimation. Also, fixing a time window led to an unfair comparison between control and cerebellar block trials, in which velocity was significantly reduced and therefore movement time was longer. Therefore, we used the peak velocity, torque impulse at the peak velocity, and maximum deviation of the hand trajectory as response measures. We have acknowledged this point in the methods section of our revised manuscript (lines 590-600). We have also refrained from using the term feedforward control throughout the text of our revised manuscript as suggested by the reviewer.
The terminology 'single-joint' movement is a bit confusing. At a minimum, it would be nice to show kinematics during different target reaches to demonstrate that certain targets are indeed single joint movements. More of an issue, however, is that it seems like these are not actually 'single-joint' movements. For example, Figure 2c shows that target 1 exhibits high elbow and shoulder torques, but in the text, T1 is described as a 'single-joint' reach (e.g. lines 155-156). The point that I think the authors are making is that these targets have low interaction torques. If that is the case, the terminology should be changed or clarified to avoid confusion.
Indeed, as reviewer #1 also noted, movements to targets 1 and 5 are not purely single-joint but rather have relatively low coupling torques. Movements to all targets involved both shoulder and elbow joints, but the degree to which each joint participated varied in a target-specific manner. In our original manuscript, we used the term “single-joint” to refer to movements in which one joint was largely stationary, resulting in minimal coupling torque at the adjacent joint. Specifically, for Targets 1 and 5, the net torque—and thus acceleration—at the elbow was negligible, causing the shoulder to experience low coupling torques (as illustrated in Figure 3c of our revised manuscript). Following this comment and to avoid confusion, we have now explained this explicitly in the revised manuscript (lines 178-187). This is supported by Supplementary Figure S2 demonstrating the net torques at the shoulder and elbow for movements to each target. We have also replaced the term ‘single-joint movements’ and ‘multi-joint movements’ with ‘movements with low coupling torques’ and ‘movements with high coupling torques’ respectively in our revised manuscript (lines 178-180, 204-207, 225-227, 230-232, 305-307, and 362-365).
The labels in Figure 3d are confusing and could use more explanation in the figure legend. In Figure 3d, it is stated that data from all monkeys is pooled. However, if there is a systematic bias between animals, this could generate spurious correlations. Were correlations also calculated for each animal separately to confirm the same trend between velocity and coupling torques holds for each animal?
We have revised the legend of Figure 3d to include a detailed explanation of how the values along each axis are computed (lines 908-920 of the revised manuscript). Please note that the pooling of data across monkeys was done after confirming that data from each animal expressed a similar trend. Specifically, the correlation coefficients were all positive but statistically significant in 3 out of the 4 monkeys. Moreover, following the reviewers’ feedback, we also did a partial correlation analysis (which controls for the variability across monkeys) and found a significant correlation (r = 0.32, p < 0.001) between reduction in peak hand velocities during cerebellar block and the net coupling torque impulse. We have updated the manuscript to include the result of the partial correlation analysis (lines 173-176).
In Table S1, it would be nice to see target-specific success rates. The data would suggest that targets with the highest interaction torques will have the largest reduction in success rates, especially during later HFS trials. Is this the case?
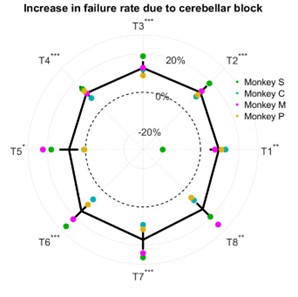
The breakdown of the percentage increase in failure rate due to cerebellar block as a function of target direction is shown in Author response image 1 inserted to this response.
Author response image 1.
Effect of cerebellar block on failure rate. The change in failure rate for the cerebellar block trials was computed relative to the control trials per session per target. The depicted values are the mean ± 95% confidence intervals across all sessions pooled from all four monkeys. The individual means of each monkey are overlaid. Statistical significance is denoted as follows: p ≥ 0.05NS, p < 0.05*, p < 0.01**, p < 0.001*** [T1-8: Targets 1-8]
The increase in failure rate due to cerebellar block was not affected by the target direction (linear mixed model analysis, target x trial-type interaction effect: p = 0.44). However, it should be noted that success/failure depends on several factors beyond just the execution related impaired limb dynamics. In a previous study (Nashef et al. 2019) we identified several causes of failure such as (i) not entering the central target in time, (ii) premature exit from the central target before the ‘go’ signal, (iii) reaction time longer than the time permitted to reach the peripheral target after the ‘go’ signal, or (iv) not holding at the peripheral target for the required time at the end of the movement.
Reviewer #3 (Recommendations for the authors):
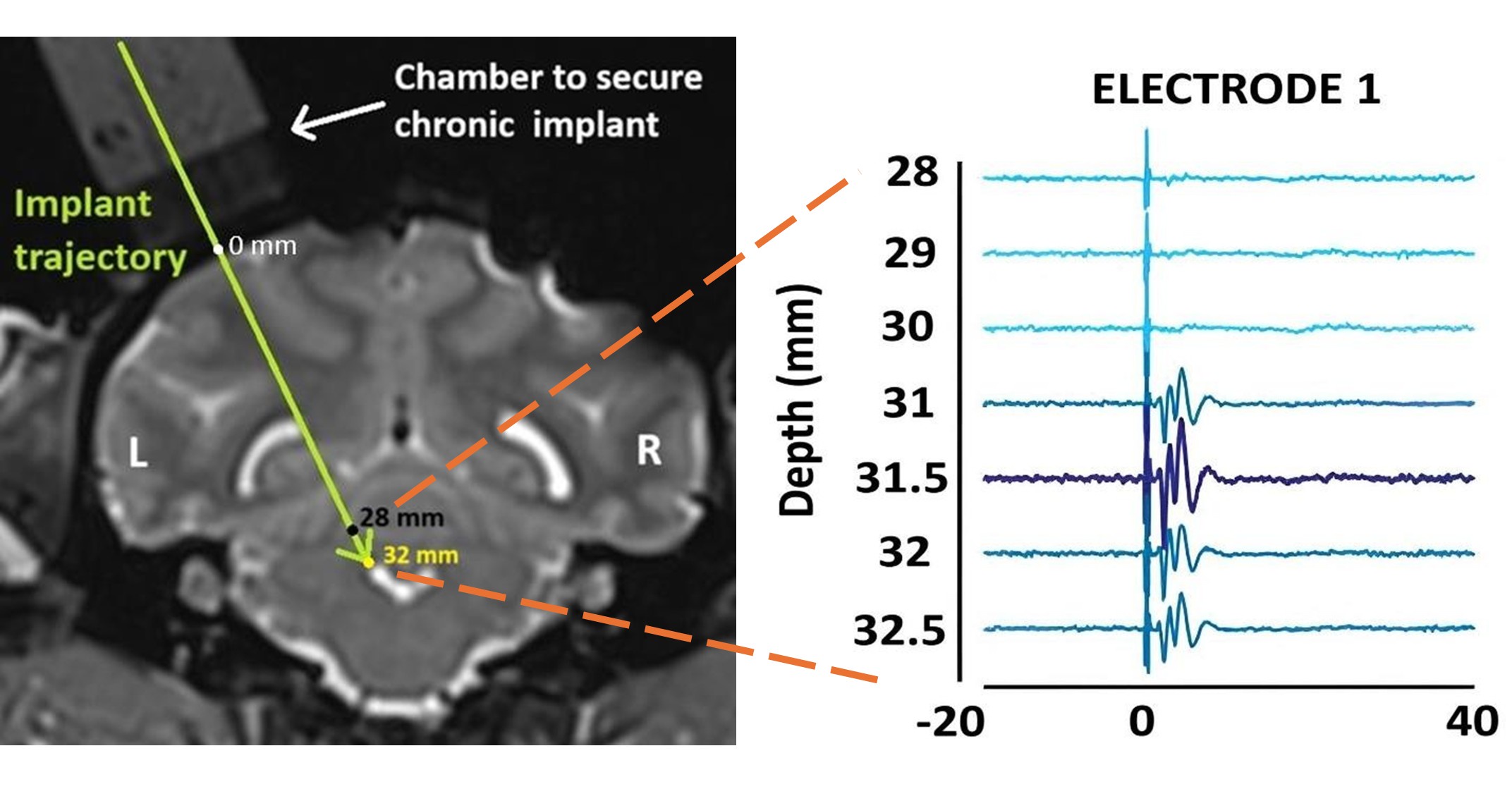
(1) It would be helpful to provide some supplemental information on electrophysiological validation of the targeting in each monkey. Was any variability in targeting observed (e.g., some targeting was more effective at eliciting cortical responses)? If so, does targeting variability relate to any of the variability in behavioral effects of HFS across monkeys?
Although we currently do not have an exact measure of the proportion of fibers blocked by HFS, our targeting approach consistently elicited robust cortical responses across monkeys. Specifically, we implanted the stimulating electrode at the location that produced the maximum peak-to-peak evoked responses in the primary motor cortex. Author response image 2 in this response demonstrates that even a slight deviation (~0.5 mm) from this optimal site reduced these responses substantially.:
Author response image 2.
Evoked responses in the primary motor cortex as a function of the location of the stimulation site. [LEFT] Coronal T2-weighted MRI showing the planned trajectory to target the superior cerebellar peduncle (location marked by the tip of the arrowhead) through a round chamber suitably positioned over the skull. [RIGHT] Evoked multi-unit (300-7500 Hz) responses from one of the recording electrodes in the primary motor cortex are used to guide the stimulating electrode to the correct implant site. As the stimulating electrode was lowered deeper, maximum peak-to-peak evoked responses were obtained at a depth of 32.5 mm relative to the cortical surface. This was chosen as the implant site. Elevating or lowering the electrode by ~0.5 mm from this depth reduced the peak-to-peak response amplitude.
(2) The emphasis in the Introduction that HFS provides direct insight into deficits seen in patients with cerebellar disease or injury is a bit overstated. Patients have very diverse etiologies, only a modest number of which might be faithfully mimicked by SCP HFS. I would suggest some text acknowledging that this is only a limited model for cerebellar disease or injury.
We agree with the reviewer that the high-frequency stimulation of the superior cerebellar peduncle provides a limited model that does not fully replicate the diverse pathologies seen in cerebellar disease or injury. In fact, in the introduction section (lines 53-59 of our revised manuscript) we have mentioned that the discrepancy in the conclusions of various clinical studies may reflect the heterogeneity of the individuals with cerebellar lesions who often have differences in lesion etiology and associated damage beyond the cerebellum itself. While this may preclude the generalization of our findings to the wider clinical population per se, our approach offers a precise and controlled method to investigate the immediate and adaptive changes in motor behavior following the disruption of cerebellar signals.
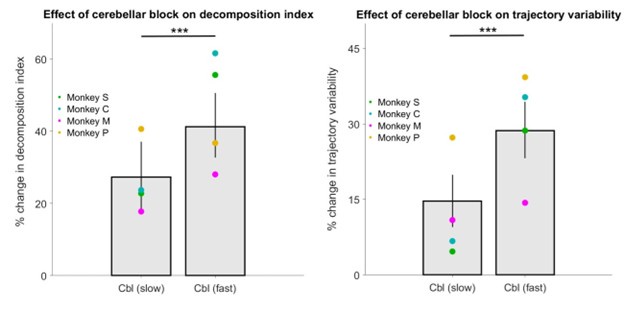
(3) Do animals with HFS show less decomposition and trajectory variability in their slower movements when compared to their faster movements? Comparisons are only made with velocity-matched control blocks, but the comparison of slower vs. faster reaches during HFS blocks would also be informative.
To answer this point we classified movements during cerebellar block as either slow or fast based on the median peak hand velocity of the cerebellar block trials per target per session. We then computed the decomposition index and trajectory variability for the fast and slow movements during cerebellar block relative to control in the same way as in Figure 5 of our manuscript (i.e., the percentage change relative to control). Our analysis revealed significantly lower movement decomposition (p < 0.001) and reduced trajectory variability (p < 0.001) for slower movements compared to faster ones within the cerebellar block condition (Author response image 3).
Author response image 3.
Effect of slow and fast movements during cerebellar block on movement decomposition and trajectory variability. [LEFT] Change in decomposition index (i.e., the proportion of the movement time during which the movement was decomposed) for slow and fast cerebellar block trials relative to all control trials. The change in median decomposition was computed per session per target and then averaged across all eight targets to arrive at one value per session. The depicted values are the mean ± 95% confidence intervals across all sessions pooled from all four monkeys. The individual means of each monkey are overlaid. [RIGHT] Change in inter-trial trajectory variability for slow and fast cerebellar block trials relative to all control trials. The trajectory variability was measured as the standard deviation of the maximum perpendicular distance of the trajectories from the Y-axis after transforming them as in Figure 5d of the main text. The change in trajectory variability for the fast and slow cerebellar block trials was then computed per session per target and averaged across all eight targets to arrive at one value per session. The depicted values are the mean ± 95% confidence intervals across all sessions pooled from all four monkeys. The individual means of each monkey are overlaid. Statistical significance is denoted as follows: p ≥ 0.05NS, p < 0.05*, p < 0.01**, p < 0.001***. [Cbl: Cerebellar block].
(4) Line 220- 'velocity' should be 'speed' or 'absolute velocity'?
The term velocity was changed to speed in the revised manuscript (line 255).
-

-

eLife Assessment
Using a unique cerebellar disruption approach in non-human primates, this study provides valuable new insight into how cerebellar inputs to the motor cortex contribute to reaching. Evidence for many claims is solid, but several analyses - especially with respect to control at the end of the reaches - could be expanded or clarified. Additional details about the behavioral task and a clearer description about the limits of the disruption approach with respect to selectivity are also warranted.
-

Reviewer #1 (Public review):
Summary:
In a previous work, Prut and colleagues had shown that during reaching, high-frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report, they extend their previous work by the addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joints. More interestingly, the experiment revealed evidence for the decomposition of the reaching movement, as well as an increase in the …
Reviewer #1 (Public review):
Summary:
In a previous work, Prut and colleagues had shown that during reaching, high-frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report, they extend their previous work by the addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joints. More interestingly, the experiment revealed evidence for the decomposition of the reaching movement, as well as an increase in the variance of the trajectory.
Strengths:
This is a rare experiment in a non-human primate that assessed the importance of cerebellar input to the motor cortex during reaching.
Weaknesses:
My major concerns are described below.
If I understand the task design correctly, the monkeys did not need to stop their hand at the target. I think this design may be suboptimal for investigating the role of the cerebellum in control of reaching because a number of earlier works have found that the cerebellum's contributions are particularly significant as the movement ends, i.e., stopping at the target. For example, in mice, interposed nucleus neurons tend to be most active near the end of the reach that requires extension, and their activation produces flexion forces during the reach (Becker and Person 2019). Indeed, the inactivation of interposed neurons that project to the thalamus results in overshooting of reaching movements (Low et al. 2018). Recent work has also found that many Purkinje cells show a burst-pause pattern as the reach nears its endpoint, and stimulation of the mossy fibers tends to disrupt endpoint control (Calame et al. 2023). Thus, the fact that the current paper has no data regarding endpoint control of the reach is puzzling to me.
Because stimulation continued after the cursor had crossed the target, it is interesting to ask whether this disruption had any effects on the movements that were task-irrelevant. The reason for asking this is because we have found that whereas during task-relevant eye or tongue movements the Purkinje cells are strongly modulated, the modulations are much more muted when similar movements are performed but are task-irrelevant (Pi et al., PNAS 2024; Hage et al. Biorxiv 2024). Thus, it is interesting to ask whether the effects of stimulation were global and affected all movements, or were the effects primarily concerned with the task-relevant movements.
If the schematic in Figure 1 is accurate, it is difficult for me to see how any of the reaching movements can be termed single joint. In the paper, T1 is labeled as a single joint, and T2-T4 are labeled as dual-joint. The authors should provide data to justify this.
Because at least part of this work was previously analyzed and published, information should be provided regarding which data are new.
-

Reviewer #2 (Public review):
This manuscript asks an interesting and important question: what part of 'cerebellar' motor dysfunction is an acute control problem vs a compensatory strategy to the acute control issue? The authors use a cerebellar 'blockade' protocol, consisting of high-frequency stimuli applied to the cerebellar peduncle which is thought to interfere with outflow signals. This protocol was applied in monkeys performing center outreaching movements and has been published from this laboratory in several preceding studies. I found the take-home-message broadly convincing and clarifying - that cerebellar block reduces muscle activation acutely particularly in movements that involve multiple joints and therefore invoke interaction torques, and that movements progressively slow down to in effect 'compensate' for these acute …
Reviewer #2 (Public review):
This manuscript asks an interesting and important question: what part of 'cerebellar' motor dysfunction is an acute control problem vs a compensatory strategy to the acute control issue? The authors use a cerebellar 'blockade' protocol, consisting of high-frequency stimuli applied to the cerebellar peduncle which is thought to interfere with outflow signals. This protocol was applied in monkeys performing center outreaching movements and has been published from this laboratory in several preceding studies. I found the take-home-message broadly convincing and clarifying - that cerebellar block reduces muscle activation acutely particularly in movements that involve multiple joints and therefore invoke interaction torques, and that movements progressively slow down to in effect 'compensate' for these acute tone deficits. The manuscript was generally well written, and the data was clear, convincing, and novel. My comments below highlight suggestions to improve clarity and sharpen some arguments.
Primary comments:
(1) Torque vs. tone: Is it known whether this type of cerebellar blockade is reducing muscle tone or inducing any type of acute co-contraction that could influence limb velocity through mechanisms different than 'atonia'? If so, the authors should discuss this information in the discussion section starting around line 336, and clarify that this motivates (if it does) the focus on 'torques' rather than muscle activation. Relatedly, besides the fact that there are joints involved, is there a reason there is so much emphasis on torque per se? If the muscle is deprived of sufficient drive, it would seem that it would be more straightforward to conceptualize the deficit as one of insufficient timed drive to a set of muscles than joint force. Some text better contextualizing the choices made here would be sufficient to address this concern. I found statements like those in the introduction "hand velocity was low initially, reflecting a primary muscle torque deficit" to be lacking in substance. Either that statement is self-evident or the alternative was not made clear. Finally, emphasize that it is a loss of self-generated torque at the shoulder that accounts for the velocity deficits. At times the phrasing makes it seem that there is a loss of some kind of passive torque.
(2) Please clarify some of the experimental metrics: Ln 94 RESULTS. The success rate is used as a primary behavioral readout, but what constitutes success is not clearly defined in the methods. In addition to providing a clear definition in the methods section, it would also be helpful for the authors to provide a brief list of criteria used to determine a 'successful' movement in the results section before the behavioral consequences of stimulation are described. In particular, the time and positional error requirements should be clear.
(3) Based on the polar plot in Figure 1c, it seemed odd to consider Targets 1-4 outward and 5-8 inward movements, when 1 and 5 are side-to-side. Is there a rationale for this grouping or might results be cleaner by cleanly segregating outward (targets 2-4) and inward (targets 6-8) movements? Indeed, by Figure 3 where interaction torques are measured, this grouping would seem to align with the hypothesis much more cleanly since it is with T2,T3,and T4 where clear coupling torques deficits are seen with cerebellar block.
4. I did not follow Figure 3d. Both the figure axis labels and the description in the main text were difficult to follow. Furthermore, the color code per animal made me question whether the linear regression across the entire dataset was valid, or would be better performed within animal, and the regressions summarized across animals. The authors should look again at this section and figure.
(5) Line 206+ The rationale for examining movement decomposition with a cerebellar block is presented as testing the role of the cerebellum in timing. Yet it is not spelled out what movement decomposition and trajectory variability have to do with motor timing per se.
-

Reviewer #3 (Public review):
Summary:
In their manuscript, "Disentangling acute motor deficits and adaptive responses evoked by the loss of cerebellar output," Sinha and colleagues aim to identify distinct causes of motor impairments seen when perturbing cerebellar circuits. This goal is an important one, given the diversity of movement-related phenotypes in patients with cerebellar lesions or injuries, which are especially difficult to dissect given the chronic nature of the circuit damage. To address this goal, the authors use high-frequency stimulation (HFS) of the superior cerebellar peduncle in monkeys performing reaching movements. HFS provides an attractive approach for transiently disrupting cerebellar function previously published by this group. First, they found a reduction in hand velocities during reaching, which was more …
Reviewer #3 (Public review):
Summary:
In their manuscript, "Disentangling acute motor deficits and adaptive responses evoked by the loss of cerebellar output," Sinha and colleagues aim to identify distinct causes of motor impairments seen when perturbing cerebellar circuits. This goal is an important one, given the diversity of movement-related phenotypes in patients with cerebellar lesions or injuries, which are especially difficult to dissect given the chronic nature of the circuit damage. To address this goal, the authors use high-frequency stimulation (HFS) of the superior cerebellar peduncle in monkeys performing reaching movements. HFS provides an attractive approach for transiently disrupting cerebellar function previously published by this group. First, they found a reduction in hand velocities during reaching, which was more pronounced for outward versus inward movements. By modeling inverse dynamics, they find evidence that shoulder muscle torques are especially affected. Next, the authors examine the temporal evolution of movement phenotypes over successive blocks of HFS trials. Using this analysis, they find that in addition to the acute, specific effects on muscle torques in early HFS trials, there was an additional progressive reduction in velocity during later trials, which they interpret as an adaptive response to the inability to effectively compensate for interaction torques during cerebellar block. Finally, the authors examine movement decomposition and trajectory, finding that even when low-velocity reaches are matched to controls, HFS produces abnormally decomposed movements and higher than expected variability in trajectory.
Strengths:
Overall, this work provides important insight into how perturbation of cerebellar circuits can elicit diverse effects on movement across multiple timescales.
The HFS approach provides temporal resolution and enables analysis that would be hard to perform in the context of chronic lesions or slow pharmacological interventions. Thus, this study describes an important advance over prior methods of circuit disruption, and their approach can be used as a framework for future studies that delve deeper into how additional aspects of sensorimotor control are disrupted (e.g., response to limb perturbations).
In addition, the authors use well-designed behavioral approaches and analysis methods to distinguish immediate from longer-term adaptive effects of HFS on behavior. Moreover, inverse dynamics modeling provides important insight into how movements with different kinematics and muscle dynamics might be differentially disrupted by cerebellar perturbation.
Weaknesses:
The argument that there are acute and adaptive effects to perturbing cerebellar circuits is compelling, but there seems to be a lost opportunity to leverage the fast and reversible nature of the perturbations to further test this idea and strengthen the interpretation. Specifically, the authors could have bolstered this argument by looking at the effects of terminating HFS - one might hypothesize that the acute impacts on muscle torques would quickly return to baseline in the absence of HFS, whereas the longer-term adaptive component would persist in the form of aftereffects during the 'washout' period. As is, the reversible nature of the perturbation seems underutilized in testing the authors' ideas.
The analysis showing that there is a gradual reduction in velocity during what the authors call an adaptive phase is convincing. That said, the argument is made that this is due to difficulty in compensating for interaction torques. Even if the inward targets (i.e., targets 6-8) do not show a deficit during the acute phase, these targets still have significant interaction torques (Figure 3c). Given the interpretation of the data as presented, it is not clear why disruption of movement during the adaptive phase would not be seen for these targets as well since they also have large interaction torques. Moreover, it is difficult to delve into this issue in more detail, as the analyses in Figures 4 and 5 omit the inward targets.
The text in the Introduction and in the prior work developing the HFS approach overstates the selectivity of the perturbations. First, there is an emphasis on signals transmitted to the neocortex. As the authors state several times in the Discussion, there are many subcortical targets of the cerebellar nuclei as well, and thus it is difficult to disentangle target-specific behavioral effects using this approach. Second, the superior cerebellar peduncle contains both cerebellar outputs and inputs (e.g., spinocerebellar). Therefore, the selectivity in perturbing cerebellar output feels overstated. Readers would benefit from a more agnostic claim that HFS affects cerebellar communication with the rest of the nervous system, which would not affect the major findings of the study.
The text implies that increased movement decomposition and variability must be due to noise. However, this assumption is not tested. It is possible that the impairments observed are caused by disrupted commands, independent of whether these command signals are noisy. In other words, commands could be low noise but still faulty.
Throughout the text, the use of the term 'feedforward control' seems unnecessary. To dig into the feedforward component of the deficit, the authors could quantify the trajectory errors only at the earliest time points (e.g., in Figure 5d), but even with this analysis, it is difficult to disentangle feedforward- and feedback-mediated effects when deficits are seen throughout the reach. While outside the scope of this study, it would be interesting to explore how feedback responses to limb perturbation are affected in control versus HFS conditions. However, as is, these questions are not explored, and the claim of impaired feedforward control feels overstated.
The terminology 'single-joint' movement is a bit confusing. At a minimum, it would be nice to show kinematics during different target reaches to demonstrate that certain targets are indeed single joint movements. More of an issue, however, is that it seems like these are not actually 'single-joint' movements. For example, Figure 2c shows that target 1 exhibits high elbow and shoulder torques, but in the text, T1 is described as a 'single-joint' reach (e.g. lines 155-156). The point that I think the authors are making is that these targets have low interaction torques. If that is the case, the terminology should be changed or clarified to avoid confusion.
The labels in Figure 3d are confusing and could use more explanation in the figure legend.
In Figure 3d, it is stated that data from all monkeys is pooled. However, if there is a systematic bias between animals, this could generate spurious correlations. Were correlations also calculated for each animal separately to confirm the same trend between velocity and coupling torques holds for each animal?
In Table S1, it would be nice to see target-specific success rates. The data would suggest that targets with the highest interaction torques will have the largest reduction in success rates, especially during later HFS trials. Is this the case?
-

Author response:
Public Reviews:
Reviewer #1 (Public review):
Summary:
In a previous work, Prut and colleagues had shown that during reaching, high-frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report, they extend their previous work by the addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joints. More interestingly, the experiment revealed evidence for the decomposition of the reaching movement, …
Author response:
Public Reviews:
Reviewer #1 (Public review):
Summary:
In a previous work, Prut and colleagues had shown that during reaching, high-frequency stimulation of the cerebellar outputs resulted in reduced reach velocity. Moreover, they showed that the stimulation produced reaches that deviated from a straight line, with the shoulder and elbow movements becoming less coordinated. In this report, they extend their previous work by the addition of modeling results that investigate the relationship between the kinematic changes and torques produced at the joints. The results show that the slowing is not due to reductions in interaction torques alone, as the reductions in velocity occur even for movements that are single joints. More interestingly, the experiment revealed evidence for the decomposition of the reaching movement, as well as an increase in the variance of the trajectory.
Strengths:
This is a rare experiment in a non-human primate that assessed the importance of cerebellar input to the motor cortex during reaching.
Weaknesses:
My major concerns are described below.
If I understand the task design correctly, the monkeys did not need to stop their hand at the target. I think this design may be suboptimal for investigating the role of the cerebellum in control of reaching because a number of earlier works have found that the cerebellum's contributions are particularly significant as the movement ends, i.e., stopping at the target. For example, in mice, interposed nucleus neurons tend to be most active near the end of the reach that requires extension, and their activation produces flexion forces during the reach (Becker and Person 2019). Indeed, the inactivation of interposed neurons that project to the thalamus results in overshooting of reaching movements (Low et al. 2018). Recent work has also found that many Purkinje cells show a burst-pause pattern as the reach nears its endpoint, and stimulation of the mossy fibers tends to disrupt endpoint control (Calame et al. 2023). Thus, the fact that the current paper has no data regarding endpoint control of the reach is puzzling to me.
We appreciate the reviewer’s point that cerebellar contributions can be particularly critical near the endpoint of a reach. In our current task design, monkeys were indeed required to hold at the target briefly—100 ms for Monkeys S and P, and 150 ms for Monkeys C and M—before receiving a reward. However, given the size of the targets and the velocity of movements, it often happened that the monkey didn’t have to stop its movement to obtain a reward. Importantly, we relaxed the task’s requirements (by increasing target size and reducing temporal constraints) to allow monkeys to perform the task under cerebellar block conditions as we found that the strict criteria in these conditions yield a low success rate. This design is suboptimal for studying endpoint accuracy which, as we now appreciate, is an important aspect of cerebellar control. In our revision, we will clarify these aspects of the task design and acknowledge that it is sub-optimal for examining the role of cerebellum in end-point control. Future studies will explicitly address this point more carefully.
Because stimulation continued after the cursor had crossed the target, it is interesting to ask whether this disruption had any effects on the movements that were task-irrelevant. The reason for asking this is because we have found that whereas during task-relevant eye or tongue movements the Purkinje cells are strongly modulated, the modulations are much more muted when similar movements are performed but are task-irrelevant (Pi et al., PNAS 2024; Hage et al. Biorxiv 2024). Thus, it is interesting to ask whether the effects of stimulation were global and affected all movements, or were the effects primarily concerned with the task-relevant movements.
This is a very interesting suggestion. Although our main analysis focused on target-directed reaching movements, we have the data for the between-trial movements under continuous stimulation (e.g., return to center movements). In our revised supplementary material, we will examine the effect of cerebellar block on endpoint velocities in inter-trial movements versus task-related movements.
If the schematic in Figure 1 is accurate, it is difficult for me to see how any of the reaching movements can be termed single joint. In the paper, T1 is labeled as a single joint, and T2-T4 are labeled as dual-joint. The authors should provide data to justify this.
The is reviewer right and movements to all targets engages shoulder and elbow but the single joint participation varied in a target-specific manner. In the manuscript, we used the term “single-joint” to indicate a target direction in which one joint remains stationary, resulting in minimal coupling torque at the adjacent joint. Specifically, for Targets 1 and 5 in our experiments, the net torque (and thus acceleration) at the elbow was negligible, and hence the shoulder experienced correspondingly low coupling torque (as illustrated in Figure 3c of our manuscript). To avoid confusion, we will use the term ‘predominantly single-joint’ movements in our revised manuscript to indicate targets with low coupling torques. We will also include an additional figure in the revised supplementary material displaying the net torques at the shoulder and elbow, similar to Figures 2c and 3c. Our goal is to demonstrate that movements to targets 1 and 5 are characterized by predominantly one-joint engagement (i.e., the elbow is stationary with low net torque) and low coupling torques, rather than implying a purely isolated, single-joint motion.
Because at least part of this work was previously analyzed and published, information should be provided regarding which data are new.
We will include a clear statement in the Methods section specifying which components of the dataset and analyses are entirely new. While some of the same animals and stimulation protocol were presented in prior work, the inverse-dynamics modeling, analyses of progressive movement changes across trials under stimulation and invariance of motor noise to movement velocity are newly reported in this manuscript.
Reviewer #2 (Public review):
This manuscript asks an interesting and important question: what part of 'cerebellar' motor dysfunction is an acute control problem vs a compensatory strategy to the acute control issue? The authors use a cerebellar 'blockade' protocol, consisting of high-frequency stimuli applied to the cerebellar peduncle which is thought to interfere with outflow signals. This protocol was applied in monkeys performing center outreaching movements and has been published from this laboratory in several preceding studies. I found the take-home-message broadly convincing and clarifying - that cerebellar block reduces muscle activation acutely particularly in movements that involve multiple joints and therefore invoke interaction torques, and that movements progressively slow down to in effect 'compensate' for these acute tone deficits. The manuscript was generally well written, and the data was clear, convincing, and novel. My comments below highlight suggestions to improve clarity and sharpen some arguments.
Primary comments:
(1) Torque vs. tone: Is it known whether this type of cerebellar blockade is reducing muscle tone or inducing any type of acute co-contraction that could influence limb velocity through mechanisms different than 'atonia'? If so, the authors should discuss this information in the discussion section starting around line 336, and clarify that this motivates (if it does) the focus on 'torques' rather than muscle activation. Relatedly, besides the fact that there are joints involved, is there a reason there is so much emphasis on torque per se? If the muscle is deprived of sufficient drive, it would seem that it would be more straightforward to conceptualize the deficit as one of insufficient timed drive to a set of muscles than joint force. Some text better contextualizing the choices made here would be sufficient to address this concern. I found statements like those in the introduction "hand velocity was low initially, reflecting a primary muscle torque deficit" to be lacking in substance. Either that statement is self-evident or the alternative was not made clear. Finally, emphasize that it is a loss of self-generated torque at the shoulder that accounts for the velocity deficits. At times the phrasing makes it seem that there is a loss of some kind of passive torque.
We appreciate the reviewer’s emphasis on distinguishing reduced muscle tone and altered co-contraction patterns as possible explanations for decreased limb velocity. Our focus on torques arises from previous studies suggesting that the core deficit in cerebellar ataxia is impaired prediction of coupling torques. This point will be added in the discussion section of our revised manuscript where we will explain why we prioritize muscle torques and how muscle-level activation collectively contributes to net joint torques. Also, we will underscore that the observed velocity deficits primarily reflect a reduction of self-generated torque at the shoulder (whether acute or adaptive), rather than any reduction in passive torques.
(2) Please clarify some of the experimental metrics: Ln 94 RESULTS. The success rate is used as a primary behavioral readout, but what constitutes success is not clearly defined in the methods. In addition to providing a clear definition in the methods section, it would also be helpful for the authors to provide a brief list of criteria used to determine a 'successful' movement in the results section before the behavioral consequences of stimulation are described. In particular, the time and positional error requirements should be clear.
Successful trials were trials in which monkeys didn’t leave the center position before the go signal and reached the peripheral target within a specific time criteria. These values varied in different monkeys. We will include detailed definitions of our success criteria in the revised methods section of our manuscript. Specifically, we will update our methods section to include (i) the timing criteria of each phase of the trials and (ii) the size of the peripheral targets indicating the tolerance for endpoint accuracy.
(3) Based on the polar plot in Figure 1c, it seemed odd to consider Targets 1-4 outward and 5-8 inward movements, when 1 and 5 are side-to-side. Is there a rationale for this grouping or might results be cleaner by cleanly segregating outward (targets 2-4) and inward (targets 6-8) movements? Indeed, by Figure 3 where interaction torques are measured, this grouping would seem to align with the hypothesis much more cleanly since it is with T2,T3,and T4 where clear coupling torques deficits are seen with cerebellar block.
We acknowledge the reviewer’s observation regarding Targets 1 and 5 being side-to-side rather than strictly “outward” or “inward.” In the first section of our results, we grouped the targets in this way to emphasize the notably stronger effect of the cerebellar block on targets involving shoulder flexion (‘outward’) as compared to those involving shoulder extension (‘inwards’). For subsequent analyses we focused on the effects of cerebellar block on outward targets where movements were single-joint (Target 1) vs. multi-joint (Targets 2-4). To clarify this aspect, in our revised manuscript we will explain the rationale for grouping T1–T4 as “outward” and T5–T8 as “inward,” including how we defined them.
(4) I did not follow Figure 3d. Both the figure axis labels and the description in the main text were difficult to follow. Furthermore, the color code per animal made me question whether the linear regression across the entire dataset was valid, or would be better performed within animal, and the regressions summarized across animals. The authors should look again at this section and figure.
We will revise the figure labels and legend to clarify how each axis is defined. Please note that pooling the data was done after confirming that data from each animal expressed a similar trend. Specifically, the correlation coefficients were all positive but statistically significant in 3 out of the 4 monkeys. Moreover, following the reviewers’ feedback, we also did a partial correlation analysis (which controls for the variability across monkeys) and found a significant correlation (r = 0.33, p < 0.001). These points will be described in the revised manuscript.
(5) Line 206+ The rationale for examining movement decomposition with a cerebellar block is presented as testing the role of the cerebellum in timing. Yet it is not spelled out what movement decomposition and trajectory variability have to do with motor timing per se.
The reviewer is right and the relations between timing, decomposition and variability need to be explicitly presented. In our revision, we will explain how decomposed movements may reflect impaired temporal coordination across multiple joints—a critical cerebellar function. We will also clarify how increased variability in joint coordination can result in increased trial-to-trial variability of trajectories.
Reviewer #3 (Public review):
Summary:
In their manuscript, "Disentangling acute motor deficits and adaptive responses evoked by the loss of cerebellar output," Sinha and colleagues aim to identify distinct causes of motor impairments seen when perturbing cerebellar circuits. This goal is an important one, given the diversity of movement-related phenotypes in patients with cerebellar lesions or injuries, which are especially difficult to dissect given the chronic nature of the circuit damage. To address this goal, the authors use high-frequency stimulation (HFS) of the superior cerebellar peduncle in monkeys performing reaching movements. HFS provides an attractive approach for transiently disrupting cerebellar function previously published by this group. First, they found a reduction in hand velocities during reaching, which was more pronounced for outward versus inward movements. By modeling inverse dynamics, they find evidence that shoulder muscle torques are especially affected. Next, the authors examine the temporal evolution of movement phenotypes over successive blocks of HFS trials. Using this analysis, they find that in addition to the acute, specific effects on muscle torques in early HFS trials, there was an additional progressive reduction in velocity during later trials, which they interpret as an adaptive response to the inability to effectively compensate for interaction torques during cerebellar block. Finally, the authors examine movement decomposition and trajectory, finding that even when low-velocity reaches are matched to controls, HFS produces abnormally decomposed movements and higher than expected variability in trajectory.
Strengths:
Overall, this work provides important insight into how perturbation of cerebellar circuits can elicit diverse effects on movement across multiple timescales.
The HFS approach provides temporal resolution and enables analysis that would be hard to perform in the context of chronic lesions or slow pharmacological interventions. Thus, this study describes an important advance over prior methods of circuit disruption, and their approach can be used as a framework for future studies that delve deeper into how additional aspects of sensorimotor control are disrupted (e.g., response to limb perturbations).
In addition, the authors use well-designed behavioral approaches and analysis methods to distinguish immediate from longer-term adaptive effects of HFS on behavior. Moreover, inverse dynamics modeling provides important insight into how movements with different kinematics and muscle dynamics might be differentially disrupted by cerebellar perturbation.
Weaknesses:
The argument that there are acute and adaptive effects to perturbing cerebellar circuits is compelling, but there seems to be a lost opportunity to leverage the fast and reversible nature of the perturbations to further test this idea and strengthen the interpretation. Specifically, the authors could have bolstered this argument by looking at the effects of terminating HFS - one might hypothesize that the acute impacts on muscle torques would quickly return to baseline in the absence of HFS, whereas the longer-term adaptive component would persist in the form of aftereffects during the 'washout' period. As is, the reversible nature of the perturbation seems underutilized in testing the authors' ideas.
We agree that our approach could more explicitly exploit the rapid reversibility of high-frequency stimulation (HFS) by examining post-stimulation ‘washout’ periods. However, for the present dataset, we ended the session after the set of cerebellar block trials. We plan to study the effect of cerebellar block on immediate post-block washout trials in the future.
The analysis showing that there is a gradual reduction in velocity during what the authors call an adaptive phase is convincing. That said, the argument is made that this is due to difficulty in compensating for interaction torques. Even if the inward targets (i.e., targets 6-8) do not show a deficit during the acute phase, these targets still have significant interaction torques (Figure 3c). Given the interpretation of the data as presented, it is not clear why disruption of movement during the adaptive phase would not be seen for these targets as well since they also have large interaction torques. Moreover, it is difficult to delve into this issue in more detail, as the analyses in Figures 4 and 5 omit the inward targets.
The reviewer is right and movements to Targets 6–8 (inward) were seemingly unaffected despite also involving significant interaction torques. In fact, we have already attempted to address this issue in the discussion section of the version 1 of our manuscript. Specifically, we note that while outward targets (2–4) tend to involve higher coupling torque impulses on average, this alone does not fully explain the differential impact of cerebellar block, as illustrated by discrepancies at the individual target level (e.g., target 7 vs. target 1). We proposed two possible explanations: (1) a bias toward shoulder flexion in the effect of cerebellar block—consistent with earlier studies showing ipsilateral flexor activation or tone changes following stimulation or lesioning of the deep cerebellar nuclei; and (2) a posture-related facilitation of inward (shoulder extension) movements from the central starting position.
The text in the Introduction and in the prior work developing the HFS approach overstates the selectivity of the perturbations. First, there is an emphasis on signals transmitted to the neocortex. As the authors state several times in the Discussion, there are many subcortical targets of the cerebellar nuclei as well, and thus it is difficult to disentangle target-specific behavioral effects using this approach. Second, the superior cerebellar peduncle contains both cerebellar outputs and inputs (e.g., spinocerebellar). Therefore, the selectivity in perturbing cerebellar output feels overstated. Readers would benefit from a more agnostic claim that HFS affects cerebellar communication with the rest of the nervous system, which would not affect the major findings of the study.
The reviewer is right that the superior cerebellar peduncle carries both descending and ascending fibers, and that cerebellar nuclei project to subcortical as well as cortical targets. However, it is also important to note that in primates the cerebellar-thalamo-cortical (CTC) pathway greatly expanded (on the expanse of the cerbello-rubro-spinal tract) in mediating cerebellar control of voluntary movements (Horne and Butler, 1995). The cerebello-subcortical pathways lost its importance over the course of evolution (Nathan and Smith, 1982, Padel et al., 1981, ten Donkelaar, 1988). In our previous study we found that the ascending spinocerebellar axons which enter the cerebellum through the SCP are weakly task-related and the descending system is quite small (Cohen et al, 2017). However, we cannot rule out an effect of HFS mediated in part through other systems. In the revised introduction section, we will clarify this point and use more careful language about the scope of our stimulation, emphasizing that HFS disrupts cerebellar communication broadly, rather than solely the cerebello-thalamo-cortical pathway.
The text implies that increased movement decomposition and variability must be due to noise. However, this assumption is not tested. It is possible that the impairments observed are caused by disrupted commands, independent of whether these command signals are noisy. In other words, commands could be low noise but still faulty.
We recognize the reviewer’s concern about linking movement decomposition and trial-to-trial trajectory variability with motor noise. As presented in our discussion section, we interpret these motor abnormalities as a form of motor noise in the sense that they are generated by faulty motor commands. We draw our interpretation from the findings of previous research work which show that the cerebellum aids in the state estimation of the limb and subsequent generation of accurate feedforward commands. Therefore, disruption of the cerebellar output may lead to faulty motor commands resulting in the observed asynchronous joint activations (i.e., movement decomposition) and unpredictable trajectories (i.e., increased trial-to-trial variability). Both observed deficits resemble increased motor noise.
Throughout the text, the use of the term 'feedforward control' seems unnecessary. To dig into the feedforward component of the deficit, the authors could quantify the trajectory errors only at the earliest time points (e.g., in Figure 5d), but even with this analysis, it is difficult to disentangle feedforward- and feedback-mediated effects when deficits are seen throughout the reach. While outside the scope of this study, it would be interesting to explore how feedback responses to limb perturbation are affected in control versus HFS conditions. However, as is, these questions are not explored, and the claim of impaired feedforward control feels overstated.
We agree that to strictly focus on feedforward control, we could have examined the measured variables in the first 50-100 ms of the movement which has been shown to be unaffected by feedback responses (Pruszynski et al. 2008, Todorov and Jordan 2002, Pruszynski and Scott 2012, Crevecoeur et al. 2013). However, in our task the amplitude of movements made by our monkeys was small and therefore the response measures we used were too small in the first 50-100 ms for a robust estimation. Also, fixing a time window led to an unfair comparison between control and cerebellar block trials, in which velocity was significantly reduced and therefore movement time was longer. Therefore, we used the peak velocity, torque-impulse at the peak velocity and maximum deviation of the hand trajectory as response measures. We will acknowledge this point in the discussion section of our revised manuscript. We will also tone down references to feedforward control throughout the text of our revised manuscript as suggested by the reviewer.
The terminology 'single-joint' movement is a bit confusing. At a minimum, it would be nice to show kinematics during different target reaches to demonstrate that certain targets are indeed single joint movements. More of an issue, however, is that it seems like these are not actually 'single-joint' movements. For example, Figure 2c shows that target 1 exhibits high elbow and shoulder torques, but in the text, T1 is described as a 'single-joint' reach (e.g. lines 155-156). The point that I think the authors are making is that these targets have low interaction torques. If that is the case, the terminology should be changed or clarified to avoid confusion.
Indeed, as reviewer #1 also noted, movements to target 1 and 5 are not purely single-joint but rather have relatively low coupling torques. Our intention while using the term “single-joint” was to indicate a target direction in which one joint remains stationary, resulting in minimal coupling torque at the adjacent joint. Specifically, for Targets 1 and 5 in our experiments, the net torque (and thus acceleration) at the elbow was negligible, and hence the shoulder experienced correspondingly low coupling torque (as illustrated in Figure 3c of our manuscript). ). To avoid confusion, we will use the term ‘predominantly single-joint’ movements in our revised manuscript to indicate targets with low coupling torques. We will also include an additional figure in the revised supplementary material displaying the net torques at the shoulder and elbow, similar to Figures 2c and 3c. Our goal is to demonstrate that movements to targets 1 and 5 are characterized by predominantly one-joint engagement (i.e., the elbow is stationary with low net torque) and low coupling torques, rather than implying a purely isolated, single-joint motion.
The labels in Figure 3d are confusing and could use more explanation in the figure legend.
In Figure 3d, it is stated that data from all monkeys is pooled. However, if there is a systematic bias between animals, this could generate spurious correlations. Were correlations also calculated for each animal separately to confirm the same trend between velocity and coupling torques holds for each animal?
We will revise the figure legend and main-text explanation for Figure 3d. Please note that pooling the data was done after confirming that data from each animal expressed a similar trend. Specifically, the correlation coefficients were positive but significant for 3 out of the 4 monkeys. Moreover, following the reviewers’ feedback, we also did a partial correlation analysis (which controls for the variability across monkeys) and found a significant correlation (r = 0.33, p < 0.001). These points will be described in the revised manuscript.
In Table S1, it would be nice to see target-specific success rates. The data would suggest that targets with the highest interaction torques will have the largest reduction in success rates, especially during later HFS trials. Is this the case?
We will provide a breakdown of the success rates as a function of targets. However, one should note that success/failure may depend on several factors beyond impaired limb dynamics. In a previous study (Nashef et al. 2019) we identified several causes of failure such as (i) not entering the central target in time, (ii) moving out too early from the peripheral target, (iii) Reaction time longer than permitted, or (iv) premature exit from the central target before permitted.
-